Cellsome® – Phosphatidylglycerol (PG)-based
Description
Plain liposomes are extensively used as artificial cell models and for studying the interaction of various types of molecules such as peptides and proteins with the surface of the lipid membranes. Plain liposomes are also used as control formulations for many different types of drug encapsulated liposome formulations. Charged liposomes are also used in many different types of blood complement studies. Plain liposomes are available in various anionic, cationic and neutral surface charges. Plain liposomes can be unilamellar or multilamellar.
Phosphatidylserine (PS) and phosphatidylglycerol (PG) are two well-known anionic molecules that form liposomes with negative zeta potential. The zeta potential of the liposomes varies depending on the molar percentage of the negatively charged lipids in the liposome formulation. Anionic Cellsome®-Phosphatidylserine (PS) and Cellsome®-Phosphatidylglycerol (PG) catalogs contain the products made from 0.5 up to 100 percent DOPS and DOPG, respectively, with various amount of negative zeta potentials.
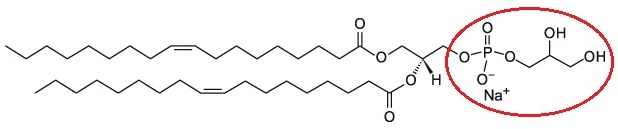
Formulation Information
Cellsome® – Phosphatidylglycerol (PG)-based
For more information on the lipid composition of the liposomes mentioned above click here.
| Buffer and Liposome Size | Specification |
|---|---|
| Buffer | Phosphate Buffered Saline* |
| pH | 7.4 |
| Liposome size | 100 nm |
| * If you prefer different buffers, please specify in your order. | |
Liposome Particle Calculator
Cellsome®-Phosphatidylglycerol (PG)-based liposomes are unilamellar and sized to 100 nm. The molar concentration of liposome is 10 mM. By having the liposome diameter (nm) and lipid concentration (µM), you can calculate the total number of the lipids in one liposome and number of the liposomes in one milliliter of the liposome solution. To use the calculator click here.
Each 100-nm liposome is composed of almost 80,000 phospholipid molecules. 10 mM liposome solution (phospholipid concentration of 10 mM) has 75.2 trillion (75.2×1012) liposome particles per milliliter of the solution. When using the liposomes calculator, you need to keep two things in mind:
- Enter the concentration in µM. As an example, 10 mM solution is 10,000 µM.
- Only include the concentration of phospholipids in your calculation. Cholesterol does not count since it inserts itself into the hydrophobic fatty acid chains of the lipid.
This calculation only works for the unilamellar liposomes which are below 200 nm in size. It does not work for larger multilamellar liposomes. For more information about the calculation see the video below:
Technical Notes
- DOPG and DOPC are both unsaturated lipids and their liquid to gel phase transition temperature is below zero degree Celsius.
- DOPG and DOPC both have unsaturated fatty acid tails. The unsaturated lipid has “kinky legs” and therefore it makes loosely packed liposomes. Saturated lipids make tightly packed liposomes. The packaging of the liposomes will impact the interaction of the molecules with the surface of the liposomes.
- Liposomes are unilamellar and sized to 100 nm. If you need them to be made in another size, then you should mention that at the time of ordering. Unilamellar liposomes can be made in 50 nm and 200 nm sizes. Liposomes that are larger than 200 nm are multilamellar structure.
- Liposomes are made in PBS buffer at pH 7.4 but it can be made in any other buffer of your choice. You can specify your buffer at the time of ordering.
- Liposomes are made in degassed buffer that is purged with argon to avoid oxidation of the unsaturated phospholipids.
- Liposomes are made under sterile condition.
- Liposomes are unilamellar and therefore due to their small size they will not settle in the bottom of the vial.
- If you need to take multiple aliquots out of the vial, for use over a period of time, then it is advised to take extreme care in not contaminating the vial. It is recommended to handle the vial under a sterile hood to maintain the sterility of the product.
- Liposomes should never be frozen. Ice crystals that form during freezing will rupture the lipid membrane of the liposomes and change the size the liposomes particles.
- Liposomes should be kept in refrigerator at 4°C in order to avoid the hydrolysis of the liposomes.
Appearance
Cellsome® – Phosphatidylglycerol (PG)-based is a white translucent liquid made of nano size unilamellar liposomes. Usually due to the small size of liposomes no settling will occur in the bottom of the vial. The liposomes are packaged in an amber vial.
Ordering/Shipping Information
- All liposome based formulations are shipped on blue ice at 4°C in insulated packages using overnight shipping or international express shipping.
- Liposomes should NEVER be frozen. Ice crystals that form in the lipid membrane can rupture the membrane and change the size of the liposomes. Liposomes in liquid form should always be kept in the refrigerator.
- Clients who order from outside of the United States of America are responsible for their government import taxes and customs paperwork. Encapsula NanoSciences is NOT responsible for importation fees to countries outside of the United States of America.
- We strongly encourage the clients in Japan, Korea, Taiwan and China to order via a distributor. Tough customs clearance regulations in these countries will cause delay in custom clearance of these perishable formulations if ordered directly through us. Distributors can easily clear the packages from customs. To see the list of the distributors click here.
- Clients ordering from universities and research institutes in Australia should keep in mind that the liposome formulations are made from synthetic material and the formulations do not require a “permit to import quarantine material”. Liposomes are NOT biological products.
- If you would like your institute’s FedEx or DHL account to be charged for shipping, then please provide the account number at the time of ordering.
- Encapsula NanoSciences has no control over delays due to inclement weather or customs clearance delays. You will receive a FedEx or DHL tracking number once your order is confirmed. Contact FedEx or DHL in advance and make sure that the paperwork for customs is done on time. All subsequent shipping inquiries should be directed to Federal Express or DHL.
Storage and Shelf Life
Storage
Cellsome® products should always be stored at in the dark at 4°C, except when brought to room temperature for brief periods prior to animal dosing. DO NOT FREEZE. If the suspension is frozen, the encapsulated drug can be released from the liposomes thus limiting its effectiveness. In addition, the size of the liposomes will also change upon freezing and thawing.
Shelf Life
Cellsome® – Phosphatidylglycerol (PG)-based is made on daily basis. The batch that is shipped is manufactured on the same day. It is advised to use the products within 4 months of the manufacturing date.











