Immunosome®-NTA(Ni) (Non-PEGylated)
SKU# IMS-2062
| Size | Price | Quantity | Subtotal |
|---|---|---|---|
| 2-ml | $600.00 | $0.00 | |
| 5-ml | $1,200.00 | $0.00 | |
| Bulk & Custom sizes | Checkout | ||
| Cart Total $0.00 | |||
Description
During the past five decades, various types of chemistries have been used for conjugation of molecules such as antibodies, peptides, proteins or other reactive ligands to the surface of liposomes. In general, the conjugation can be achieved through the N-terminus, the C-terminus or the available sulfur (e.g. Fab’ fraction or thiolated antibodies). Not all chemistries have the same yield and efficiency of conjugation and often reproducing biocompatible batches can be a challenge.
It is well established that electron-rich ligands such as histidine, tryptophan or cysteine show a relatively high affinity to bond with electropositive transition metals, including Co+2, Ni+2, Cu+2 and Zn+2. This observation has been exploited to improve and control the association of diverse histidine-tagged peptides to liposomes containing metal-chelating lipids. Therefore, immunoliposomes can be generated using nickel-chelating lipids such as Ni-nitrilotriacetic acid (NTA) and 1,2-dioleoyl-sn-glucero-3-[N-(amino-1-carboxypentyl)-iminodiacetic acid) succinyl] (DOGS-NTA aka DGS-NTA), which are commercially available, and His-tagged proteins or peptides. When the liposomes with Ni-NTA headgroups are combined with the His residues, typically at the N- or C-terminus of proteins, the proteins reversibly anchor to the liposomes.
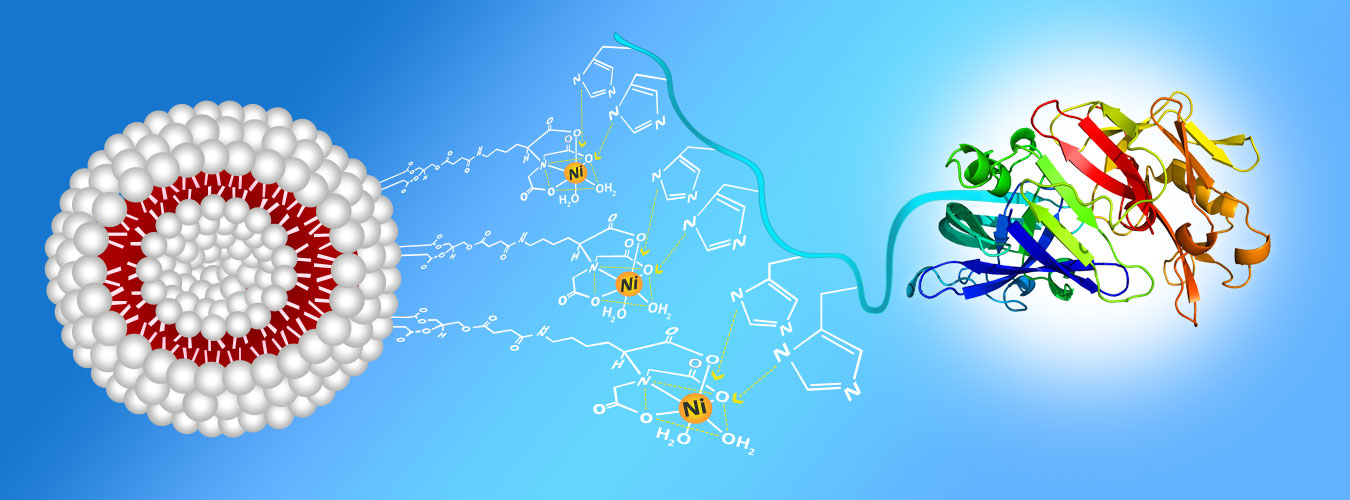
Immunosome®-NTA(Ni) is a non-PEGylated product. For the other reactive (PEGylated and non-PEGyalated products) Immunosome® products suitable for other types of conjugation method see here.
Download Product Insert Download Safety Datasheet (SDS)
Formulation Information
Immunosome®-NTA(Ni) (Non-PEGylated)
| Lipid Composition | Concentration (mg/ml) | Concentration (mM) | Molar Ratio Percentage |
|---|---|---|---|
| L-alpha-Phosphatidylcholine | 12 | 15.5 | 69 |
| Cholesterol | 2.6 | 6.73 | 30 |
1,2-Dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl] (nickel salt)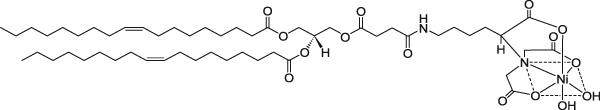 | 0.24 | 0.22 | 1 |
| Total | 14.84 mg/ml | 22.45 mM | 100 |
| Buffer and Liposome Size | Specification |
|---|---|
| Buffer | Phosphate Buffered Saline |
| pH | 7.4 |
| Liposome Size | 100 nm |
Conjugation Protocol
Materials and Equipment
In order to conjugate your antibody or protein tagged with FBP to Immunosome®-NTA(Ni) liposomes you will need:
- Laboratory vortex mixer is recommended to have.
- Laboratory magnetic stirrer is needed for dialysis.
- Float-A-Lyzer® with a proper MWCO that easily allows the cleanup of your liposome conjugated ligand from free and non-conjugated protein/peptide/ligand. You need to make sure that the MWCO is below 1,000,000 dalton. At 1,000,000 dalton, the pore size on the dialysis membrane gets close to 100 nm and therefore your liposomes can be dialyzed out. You cannot use dialysis cassettes blindly. Please understand the technique before using either spin column or dialysis cassette. If you do not use the correct MWCO you can lose your entire prep. For this protocol we recommend MWCO of 300,000 dalton.
Preparation Method
- The total lipid concentration in Immunosome®-NTA(Ni) is 22.45 mM. 1% mol of the lipid in liposomes contains NTA(Ni) group and only half of them are exposed to the outside of the liposomes, which is equal to 0.11 mM of reactive conjugable lipid. For the 2 ml volume liposomes, this is equal to 2.20×10-7 mol, and for the 5 ml volume liposomes this is equal to 5.50×10-7 mol of NTA(Ni). Add 1:1 molar ratio of His6-tagged protein, peptide or antibody to NTA(Ni) lipid. Incubate Immunosome®-NTA(Ni) with His6-tagged antibody, protein, peptide or ligand for 1 hour at room temperature.
- Remove the non-conjugated antibody, protein, peptide or ligand by dialysis. We prefer dialysis to size exclusion columns. Dialysis is a much slower process but there will be minimum loss of immunoliposomes after the prep is cleaned from non-conjugated protein/peptide/ligand. Spin columns are much fast, but you can easily lose over 50% of the liposomes on the spin column. We recommend using Float-A-Lyzer® dialysis cassette from Spectrum Labs. You need to choose a cassette with proper MWCO depending on the MW of your protein, ligand, antibody or antibody fragment. NOTE: If you decide to use a dialysis cassette, you need to make sure that the MWCO is below 1,000,000 dalton. At 1,000,000 dalton, the pore size on the dialysis membrane gets close to 100 nm and therefore your liposomes can be dialyzed out. You cannot use dialysis cassettes and spin columns blindly. They come in various sizes and you need to choose the correct size wisely. Dialyze the immunoliposome solution in 1 liter of PBS at pH 7.4 for 8 hours. Change the dialysis buffer with a fresh 1 liter of PBS and let is dialyze for another 8 hours. After this step your cleaned up immunoliposome is ready to be used.re using either spin column or dialysis cassette. If you do not use the correct MWCO you can lose your entire prep.
Liposome Particle Calculator
Immunosomes are unilamellar liposomes and sized to 100 nm. The molar concentration of liposome is 22.45 mM. By having liposome diameter (nm) and lipid concentration (µM), you can calculate the total number of the lipids in one liposome and the number of the liposomes in one milliliter of the liposome solution. To use the calculator click here.
Technical Notes
- Higher concentration of DOGS-Ni-NTA lipid in the liposome formulation will cause an increased change of nonspecific binding and therefore it is important to keep the molar percentage of chelating DOGS-Ni-NTA lipid below 3% to avoid nonspecific binding. Immunosome®-Ni-NTA and Immunodox®Ni-NTA kits all contain 1% DOGS-Ni-NTA which is an optimized concentration for specific binding to His6-tagged proteins, peptides and antibody fragments.
- The binding reaction between a His6-tagged peptide and DOGS-NTA-Ni liposomes will be inhibited in the presence of high concentration of imidazole (~166 mM).
- It should be noted that the use of a 10-histidine tag promoted liposome–liposome crosslinking in the absence of PEG-modified lipids. This could be explained by suggesting that the stretch of 10 histidine residues would be sufficient to bind more than one DOGS-NTA-Ni liposome.
- It has been demonstrated that the presence of PEG polymers inhibits surface reactions between liposomes, and thus can be used to prevent aggregation.
- If you are using a ligand or peptide that is hydrophobic then it is recommended to solubilize it in DMSO or DMF and then add the buffer to it. It is recommended not to use more than 5% volume of DMSO or DMF in the solution. DMF and DMSO are both compatible with liposomes and they are also miscible in water. Other organic solvent such as ethanol and chloroform are not compatible with liposomes and will cause the liposomes to lyse. If you end up using DMSO or DMF then after the conjugation reaction is done, you need to remove DMSO and DMF from the liposomes. In order to do that you need to use a dialysis cassette that is made from REGENERATED CELLULOSE MEMBRANE. NOTE: Not all membranes are compatible with DMF and DMSO. We recommend using a Slide-A-Lyzer™ MINI Dialysis Device with MWCO of 2K made from regenerated cellulose membrane manufactured by ThermoFisher. After DMSO or DMF is removed you can use Float-A-Lyzer® dialysis device for the final step of cleaning up the prep.
- Liposomes should be kept at 4°C and NEVER be frozen.
Database
Direct link to the database page for easy navigation: Immunoliposomes Conjugation Database
Appearance
Immunosome®-NTA(Ni) is a white translucent liquid made of nano size unilamellar liposomes. Usually due to the small size of liposomes no settling will occur in the bottom of the vial. The liposomes are packaged in an amber vial.
Ordering/Shipping Information
- All liposome based formulations are shipped on blue ice at 4°C in insulated packages using overnight shipping or international express shipping.
- Liposomes should NEVER be frozen. Ice crystals that form in the lipid membrane can rupture the membrane, change the size of the liposomes and cause the encapsulated drug to leak out. Liposomes in liquid form should always be kept in the refrigerator.
- Clients who order from outside of the United States of America are responsible for their government import taxes and customs paperwork. Encapsula NanoSciences is NOT responsible for importation fees to countries outside of the United States of America.
- We strongly encourage the clients in Japan, Korea, Taiwan and China to order via a distributor. Tough customs clearance regulations in these countries will cause delay in custom clearance of these perishable formulations if ordered directly through us. Distributors can easily clear the packages from customs. To see the list of the distributors click here.
- Clients ordering from universities and research institutes in Australia should keep in mind that the liposome formulations are made from synthetic material and the formulations do not require a “permit to import quarantine material”. Liposomes are NOT biological products.
- If you would like your institute’s FedEx or DHL account to be charged for shipping, then please provide the account number at the time of ordering.
- Encapsula NanoSciences has no control over delays due to inclement weather or customs clearance delays. You will receive a FedEx or DHL tracking number once your order is confirmed. Contact FedEx or DHL in advance and make sure that the paperwork for customs is done on time. All subsequent shipping inquiries should be directed to Federal Express or DHL.
Storage and Shelf Life
Storage
Immunosome® products should always be stored at in the dark at 4°C, except when brought to room temperature for brief periods prior to animal dosing. DO NOT FREEZE. If the suspension is frozen, the encapsulated drug can be released from the liposomes thus limiting its effectiveness. In addition, the size of the liposomes will also change upon freezing and thawing.
Shelf Life
Immunosome®-NTA(Ni) is made on daily basis. The batch that is shipped is manufactured on the same day. It is advised to use the products within 4 months of the manufacturing date.











