ImmunoFluor™-PDP (Non-PEGylated)
Description
During the past five decades, various types of chemistries have been used for conjugation of molecules such as antibodies, peptides, proteins or other reactive ligands to the surface of liposomes. In general, the conjugation can be achieved through the N-terminus, the C-terminus or the available sulfur (e.g. Fab’ fraction or thiolated antibodies). Not all chemistries have the same yield and efficiency of conjugation and often reproducing biocompatible batches can be a challenge. The liposomes containing pyridyldithiopropionate (PDP) lipids are used to conjugate proteins, antibodies and other molecules containing the reactive moiety. PDP lipids are not as widely used as maleimide lipids, but they do have their own niche application. The PDP group contains disulfide, which can react with sulfhydryl or thiolated proteins/antibodies. Therefore, PDP-functionalized liposomes can be used in two ways:
Method A. In this approach, the pyridyldithio group of the PDP is first reduced by a reducing agent (dithiothreitol, DTT). Maleimide-containing antibodies are then efficiently coupled to the surface of liposomes. The thiol-maleimide procedure is one of the most desirable reactions in bioconjugate chemistry due to its simplicity and high coupling efficiency in aqueous solution. The reaction, which is based on the stable thioether linkage between a thiol group (reduced form of PDP-liposome) and the corresponding maleimide group, occurs selectively and irreversibly at neutral pH (6.5-7.5), and the formed bonds are not cleaved by reducing agents. In addition, due to the presence of two different oxidation states of sulfur residues (oxidized and reduced states as a disulfide bond and sulfhydryl group, respectively) on the two conjugating components (i.e., the liposome and protein/antibody), the probability of the crosslinking of the homologous agents is low. Therefore, protein-protein and liposome-liposome crosslinking does not usually happen.
Method B. Alternatively, the PDP group can participate in disulfide exchange reactions with thiols present on targeting proteins/antibodies. The coupling reaction is fast and conducted under mild conditions. However, the formed disulfide bonds have been reported to be less stable than thioether bonds. Moreover, even in an alkaline medium (pH 8.0), thiol groups are oxidized. The disulfide bond formed between the protein/antibody and liposomes can also be broken in the presence of a reducing agent and therefore, the conjugation reaction is reversible.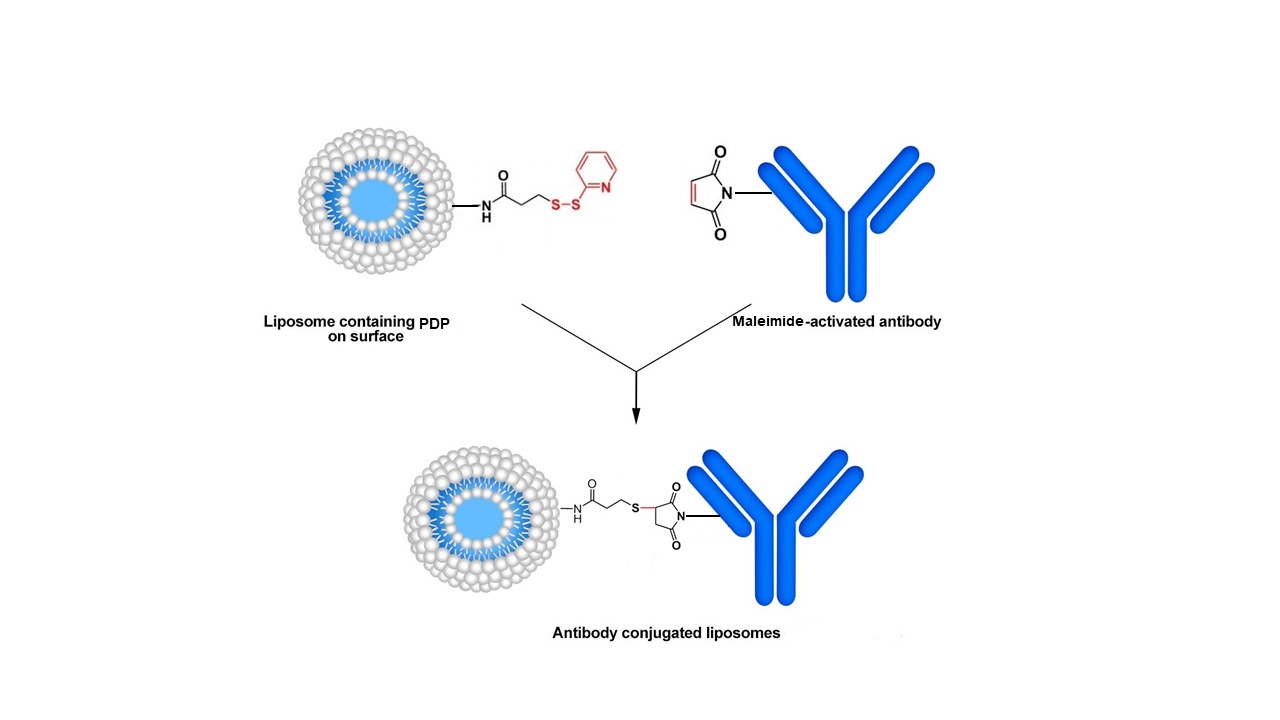
Method A. Conjugation of maleimide-modified antibody to a PDP-modified liposome.
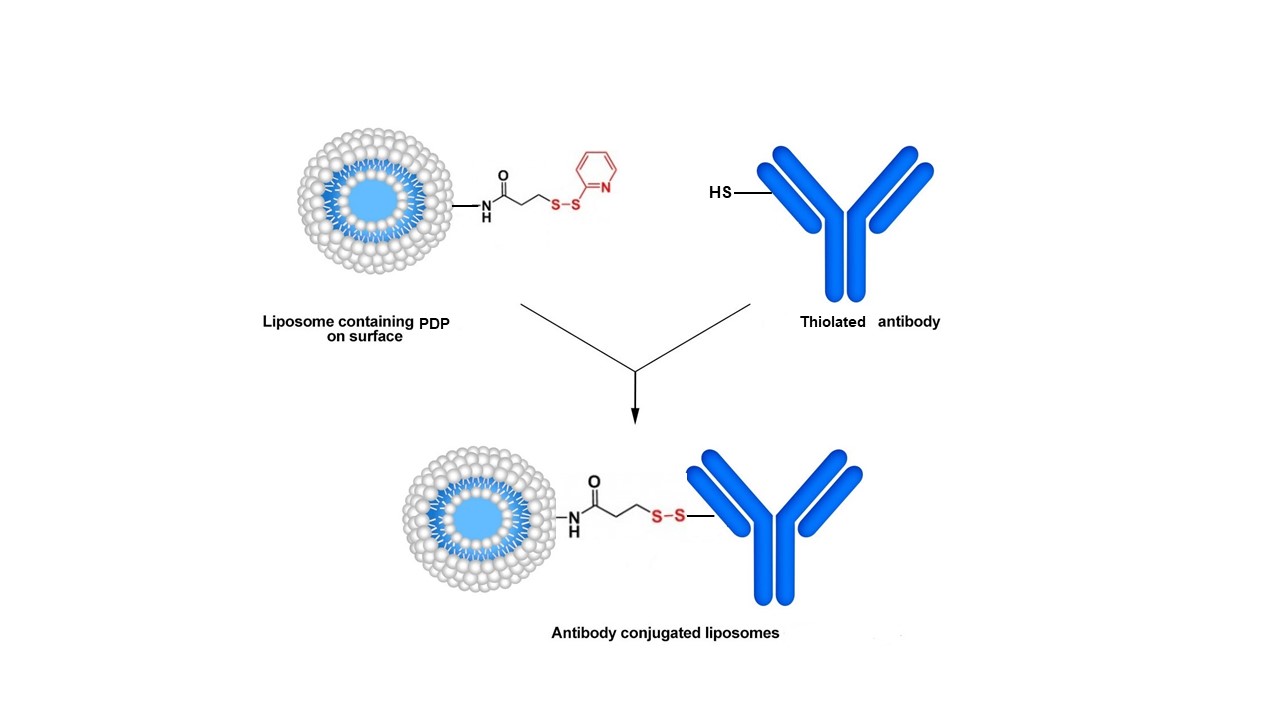
Method B. Conjugation of a thiol-modified antibody to a PDP-modified liposome.
ImmunoFluor™-PDP is a non-PEGylated product. For other reactive (PEGylated and non-PEGyalated products) ImmunoFluor™ products suitable for other types of conjugation method see here.
Formulation Information
ImmunoFluor™-PDP (Non-PEGylated)
| Lipid Composition | Concentration (mg/ml) | Concentration (mM) | Molar Ratio Percentage |
|---|---|---|---|
| L-alpha-Phosphatidylcholine | ~12 | ~15.5 | ~69 |
| Cholesterol | 2.6 | 6.73 | 30 |
1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine-N-[3-(2-pyridyldithio)propionate]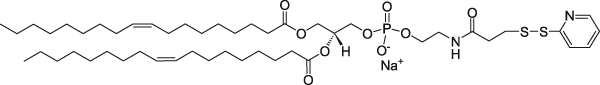 | 0.22 | 0.22 | 1 |
| Fluorescent Lipid (see below) | Varies based on the dye | Varies based on the dye | Varies based on the dye |
| Total | ~14.82 mg/ml | ~22.45 mM | 100 |
| Fluorescent Dye | Excitation/Emission (nm) | Molecular Structure |
|---|---|---|
| 1,1'-Dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI) | 549/565 |  |
| 3,3'-Dilinoleyloxacarbocyanine perchlorate (DiO) | 484/501 | 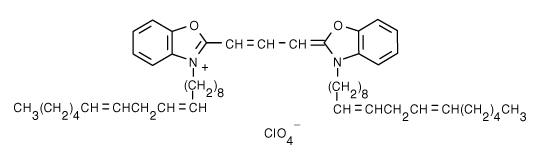 |
| 1,1'-Dioctadecyl-3,3,3',3'-tetramethylindodicarbocyanine, 4-chlorobenzenesulfonate salt (DiD) | 644/665 | 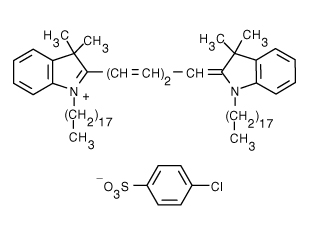 |
| 1,1'-Dioctadecyl-3,3,3',3'-tetramethylindotricarbocyanine iodide (DiR) | 750/780 | 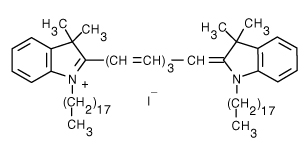 |
| 4-(4-(Dihexadecylamino)styryl)-N-methylpyridinium iodide (DiA) | 456/590 | 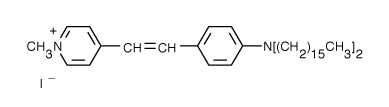 |
| 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2-1,3-benzoxadiazol-4-yl) (ammonium salt) (NBD on head group) | 460/535 | 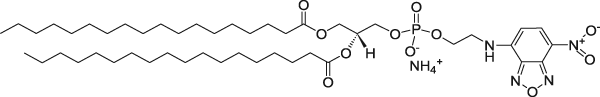 |
| 1-Palmitoyl-2-{12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]dodecanoyl}-sn-glycero-3-phosphocholine (NBD on fatty acid tail) | 460/534 | 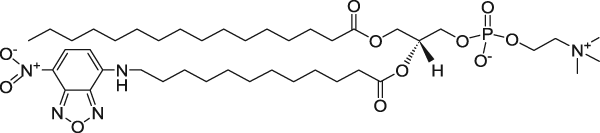 |
| 1,2-Dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (ammonium salt) (Rhodamine lipid) | 560/583 | 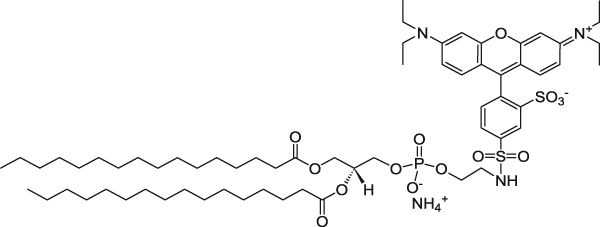 |
| 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine-N-(5-dimethylamino-1-naphthalenesulfonyl) (ammonium salt) (Dansyl lipid) | 336/513 | 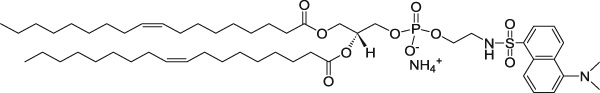 |
| 1,2-Dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(1-pyrenesulfonyl) (ammonium salt) (Pyrene lipid) | 351/379 | 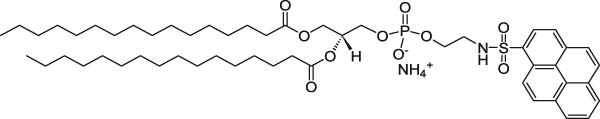 |
| Buffer and Liposome Size | Specification |
|---|---|
| Buffer | Phosphate Buffered Saline |
| pH | 7.4 |
| Liposome Size | 100 nm |
Conjugation Protocol
Materials and Equipment
- Laboratory vortex mixer is recommended to have.
- Laboratory magnetic stirrer is needed for dialysis.
- Float-A-Lyzer® with a proper MWCO that easily allows the cleanup of your liposome conjugated ligand from free and non-conjugated protein/peptide/ligand. You need to make sure that the MWCO is below 1,000,000 dalton. At 1,000,000 dalton, the pore size on the dialysis membrane gets close to 100 nm and therefore your liposomes can be dialyzed out. You cannot use dialysis cassettes blindly. Please understand the technique before using either spin columns or dialysis cassettes. If you do not use the correct MWCO, you can lose your entire prep. For this protocol, we recommend MWCO of 300,000 dalton.
- Sephadex® G-15 column.
- DL-Dithiothreitol (DTT) (for method A).
- Ethylenediaminetetraacetic acid (EDTA) (for method B).
Preparation Method
Method A
- The total lipid concentration in Immunosome®-PDP is 22.45 mM. 1% mol of the lipid in liposomes contains PDP group and only half of them are exposed to the outside of the liposomes, which is equal to 0.11 mM of reactive conjugable lipid. For 2 ml volume liposome, this is equal to 2.20×10-7 mol of PDP. To reduce the disulfide linkage, incubate the liposome containing PDP with DTT solution (liposome-PDP:DTT molar ratio of 1:250) to a final concentration of 20 mM for 30 min at room temperature.
- Separate DTT by passing the liposomes over a Sephadex® G-15 column eluted with 25 mM HEPES, 25 mM MES, 140 mM NaCl, pH 6.7 buffer.
- Immediately after removing DTT, incubate the thiolated liposomes with the maleimide-modified protein/antibody at a ligand:liposome molar ratio of 1:1000 (ligand:active lipid, 1:10 molar ratio) for overnight at room temperature.
- The free thiols on the liposome surface are blocked by incubation the suspension with iodoacetamide (0.2 mM) for 2 h at room temperature.
- Remove the non-conjugated protein, peptide or antibody from the immunoliposomes by dialysis. We prefer dialysis to size exclusion columns. Dialysis is a much slower process but there will be minimum loss of immunoliposomes after the prep is cleaned from non-conjugated protein/peptide/ligand. Spin columns are much faster; however, you can easily lose over 50% of the liposomes on the spin column. We recommend using Float-A-Lyzer® dialysis cassette from Spectrum Labs. You will need to choose a cassette with proper MWCO depending on the MW of your protein, peptide, antibody or antibody fragment. NOTE: If you decide to use a dialysis cassette, you will need to make sure that the MWCO is below 1,000,000 dalton. At 1,000,000 dalton, the pore size on the dialysis membrane gets close to 100 nm and therefore, your liposomes can be dialyzed out. You cannot use dialysis cassettes and spin columns blindly. They come in various sizes and you need to choose the correct size wisely. Dialyze the immunoliposome solution in 1 liter of PBS at pH 7.4 for 8 hours. Change the dialysis buffer with a fresh 1 liter of PBS and let is dialyze for another 8 hours. After this step, your cleaned up immunoliposome is ready to be used.
Method B
- The total lipid concentration in Immunosome®-PDP is 22.45 mM. 1% mol of the lipid in liposomes contains PDP group and only half of them are exposed to the outside of the liposomes, which is equal to 0.11 mM of reactive conjugable lipid. For 2 ml volume liposome, this is equal to 2.20×10-7 mol of PDP. Add the sulfhydryl-containing protein/antibody to PDP-liposome at 1:1000 molar ratio (ligand:active lipid, 1:10 molar ratio). In order to prevent metal-catalyzed oxidation of sulfhydryl, the reaction should be done in the presence of 10 mM EDTA. The pH is adjusted to 8.0 and the coupling reaction allowed to proceed overnight at room temperature with stirring under nitrogen or argon atmosphere to prevent lipid oxidation.
- Remove the non-conjugated protein, peptide or antibody from the immunoliposomes by dialysis. We prefer dialysis to size exclusion columns. Dialysis is a much slower process but there will be minimum loss of immunoliposomes after the prep is cleaned from non-conjugated protein/peptide/ligand. Spin columns are much faster; however, you can easily lose over 50% of the liposomes on the spin column. We recommend using Float-A-Lyzer® dialysis cassette from Spectrum Labs. You will need to choose a cassette with proper MWCO depending on the MW of your protein, peptide, antibody or antibody fragment. NOTE: If you decide to use a dialysis cassette, you will need to make sure that the MWCO is below 1,000,000 dalton. At 1,000,000 dalton, the pore size on the dialysis membrane gets close to 100 nm and therefore, your liposomes can be dialyzed out. You cannot use dialysis cassettes and spin columns blindly. They come in various sizes and you need to choose the correct size wisely. Dialyze the immunoliposome solution in 1 liter of PBS at pH 7.4 for 8 hours. Change the dialysis buffer with a fresh 1 liter of PBS and let is dialyze for another 8 hours. After this step, your cleaned up immunoliposome is ready to be used.
Quantification of reactive sulfhydryl in antibodies or ligands (Ellman’s Assay)
The yield of conjugation is the most important factor in formulating immunoliposomes. Many scientists simply assume that their thiolated antibody or the Fab’ fraction contains reactive sulfhydryl for conjugation to maleimide lipid without further assaying. Disulfide bridge can form very easily so it is very important to quantify the available reactive sulfhydryl in your antibody or ligand solution before performing the conjugation reaction with maleimide liposomes.
Ellman’s assay is a widely used assay for determining the amount of free sulfhydryl. You can follow the step by step protocol here.
Liposome Particle Calculator
Immunosomes are unilamellar liposomes and sized to 100 nm. The molar concentration of liposome is 22.45 mM. By having liposome diameter (nm) and lipid concentration (µM), you can calculate the total number of the lipids in one liposome and the number of the liposomes in one milliliter of the liposome solution. To use the calculator click here.
Technical Notes
- After conjugation reactions, liposomes containing excess maleimide or thiol groups may exhibit undesirable qualities, such as aggregation, reactions in vitro and in vivo, and immunogenicity. These reactive moieties can be quenched with reagents containing iodo-, maleimide, or sulfhydryl groups where appropriate. This is likely to be a particularly serious problem for thiolated liposomes. Therefore, it is recommended that the antibody be thiolated in order to generate the appropriate reactive entities for the final conjugation reaction.
- In order to prevent oxidation of sulfhydryl on antibody and formation of disulfide bridge, the coupling reaction must be performed under an inert atmosphere such as argon or nitrogen. To set up a inert gas chamber we recommend using Aldrich®-Atmosbag with is a flexible, inflatable polyethylene chamber with built-in gloves which is a portable and inexpensive alternative to laboratory glove box.
- Maleimide group on lipid is highly sensitive of alkaline pH and it will hydrolyze rapidly at higher pH. Experimental investigations have been shown that in alkaline condition (pH > 7.5), maleimide and its derivatives are hydrolyzed to a non-reactive maleamic acid (see the figure below). This instability should be considered in any quantitative procedures, such as coupling with sulfhydryl groups. Therefore, it is very important to make sure that the pH of the reaction with stay between 6.5 and 7 during the entire process.
- If your goal is to conjugate a thiolated protein/antibody containing reactive sulfhydryl to liposomes to form an immunoliposome, it is recommended to use liposomes containing maleimide reactive lipids.
- The amount of the maleimide-activated protein/antibody bound per liposome in Method A depends on the number of free thiols on the liposome surface (formed in step 1) and the reaction efficiency increase with increasing PDP/mAb molar ratio in the incubation mixture.
- If you are using a ligand or peptide that is hydrophobic then it is recommended to solubilize it in DMSO or DMF and then add the buffer to it. It is recommended not to use more than 5% volume of DMSO or DMF in the solution. DMF and DMSO are both compatible with liposomes and they are also miscible in water. Other organic solvent such as ethanol and chloroform are not compatible with liposomes and will cause the liposomes to lyse. If you end up using DMSO or DMF then after the conjugation reaction is done, you need to remove DMSO and DMF from the liposomes. In order to do that you need to use a dialysis cassette that is made from REGENERATED CELLULOSE MEMBRANE. NOTE: Not all membranes are compatible with DMF and DMSO. We recommend using a Slide-A-Lyzer™ MINI Dialysis Device with MWCO of 2K made from regenerated cellulose membrane manufactured by ThermoFisher. After DMSO or DMF is removed you can use Float-A-Lyzer® dialysis device for the final step of cleaning up the prep.
- Liposomes should be kept at 4°C and NEVER be frozen.
Database
Direct link to the database page for easy navigation: Immunoliposomes Conjugation Database
Appearance
ImmunoFluor™-PDP formulation is colored and the color depends on the type of the fluorescent dye that is used (see SDS for appearance). Usually due to the small size of liposomes no settling will occur in the bottom of the vial. The liposomes are packaged in an amber vial.
Ordering/Shipping Information
- All liposome based formulations are shipped on blue ice at 4°C in insulated packages using overnight shipping or international express shipping.
- Liposomes should NEVER be frozen. Ice crystal that form in the lipid membrane can rupture the membrane, change the size of the liposomes and cause the encapsulated drug to leak out. Liposomes in liquid form should always be kept in the refrigerator.
- Clients who order from outside of the United States of America are responsible for their government import taxes and customs paperwork. Encapsula NanoSciences is NOT responsible for importation fees to countries outside of the United States of America.
- We strongly encourage the clients in Japan, Korea, Taiwan and China to order via a distributor. Tough customs clearance regulations in these countries will cause delay in custom clearance of these perishable formulations if ordered directly through us. Distributors can easily clear the packages from customs. To see the list of the distributors click here.
- Clients ordering from universities and research institutes in Australia should keep in mind that the liposome formulations are made from synthetic material and the formulations do not require a “permit to import quarantine material”. Liposomes are NOT biological products.
- If you would like your institute’s FedEx or DHL account to be charged for shipping, then please provide the account number at the time of ordering.
- Encapsula NanoSciences has no control over delays due to inclement weather or customs clearance delays. You will receive a FedEx or DHL tracking number once your order is confirmed. Contact FedEx or DHL in advance and make sure that the paperwork for customs is done on time. All subsequent shipping inquiries should be directed to Federal Express or DHL.
Storage and Shelf Life
Storage
ImmunoFluor™ products should always be stored at in the dark at 4°C, except when brought to room temperature for brief periods prior to animal dosing. DO NOT FREEZE. If the suspension is frozen, the encapsulated drug can be released from the liposomes thus limiting its effectiveness. In addition, the size of the liposomes will also change upon freezing and thawing.
Shelf Life
ImmunoFluor™ (Non-PEGylated) is made on daily basis. The batch that is shipped is manufactured on the same day. It is advised to use the products within 2 months of the manufacturing date.
References and background reading
6. Torchilin V, Weissig V. Liposomes: a practical approach. Oxford University Press; 2003 Jun 5.











