ImmunoFluor™-NHS (PEGylated) (Post-insertion)
Description
Numerous techniques have been developed to prepare immunoliposomes based on the nucleophilic reactivity of free amine groups of proteins or peptides. However, the most common, versatile and straightforward activation chemistry for creating reactive acylating reagents and labeling peptides/proteins is to form NHS ester with primary amines. A single step nucleophilic substitution reaction between NHS ester derivative and alpha amines at the N terminal or the beta amines of lysine side chains leads to the formation of a stable amide bond.
N-Hydroxysuccinimide (NHS) esters of DSPE-PEG-NHS liposomes react with the primary amine groups on the peptides, proteins, antibodies or other functional ligands for targeted drug delivery applications. The nucleophilic attack of the amine groups on the NHS-activated carbonyl group of the DSPE-PEG-NHS results in the elimination of the NHS group and formation of amide linkage.
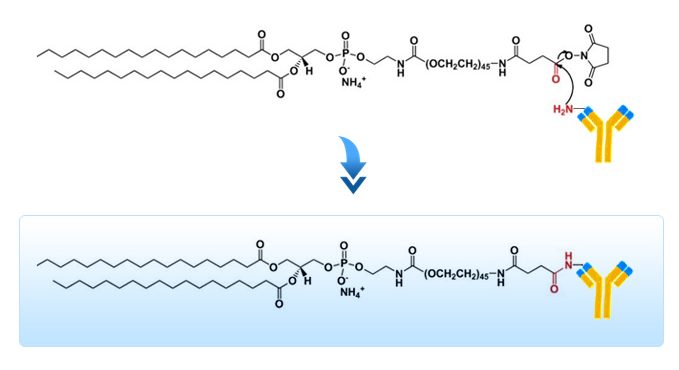
ImmunoFluor™-NHS is a PEGylated product. For other amine reactive (PEGylated and non-PEGyalated) products and also ImmunoFluor™ products suitable for other types of conjugation methods see here.
FORMULATION INFORMATION
ImmunoFluor™-NHS (PEGylated) (Post-insertion)
| Post Insertion Kit (3 Vials) | Specification |
|---|---|
| Vial 1 | Preformed liposomes composed of HSPC:Cholesterol:Fluorescent Lipid (59.5:40:0.5 molar ratio) |
| Vial 2 | DSPE-PEG(2000)-NHS lipid (reactive PEGylated lipid) in powder form |
| Vial 3 | DSPE-PEG(2000) lipid (non-reactive PEGylated lipid) in powder form |
| Lipid Composition for Vial 1* | Concentration (mg/ml) | Concentration (mM) | Molar Ratio Percentage |
|---|---|---|---|
| Hydrogenated Soy PC | ~11.5 | ~14.66 | ~60 |
| Cholesterol | 3.83 | 9.9 | 40 |
| Fluorescent Lipid (see below) | Varies based on the dye | Varies based on the dye | Varies based on the dye |
| Total | ~15.33 mg/ml | ~24.56 mM | 100 |
| * For the 5-ml kit, the volume of vial 1 is 4 ml. 1 ml of micelle solution that are formed using vials 2 and 3 will be added to this vial to make the final volume of 5 ml in the final product. For the 2-ml kit, the volume of vial 1 is 1.6 ml. 0.4 ml of micelle solution that is formed using vials 2 and 3 will be added to this vial to make the final volume of 2 ml in the final product. | |||
| Fluorescent Dye | Excitation/Emission (nm) | Molecular Structure |
|---|---|---|
| 1,1'-Dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI) | 549/565 | 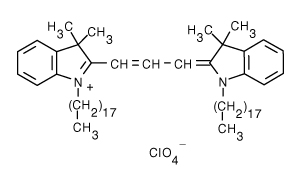 |
| 3,3'-Dilinoleyloxacarbocyanine perchlorate (DiO) | 484/501 | 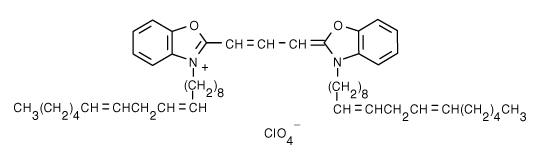 |
| 1,1'-Dioctadecyl-3,3,3',3'-tetramethylindodicarbocyanine, 4-chlorobenzenesulfonate salt (DiD) | 644/665 | 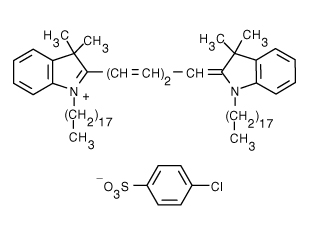 |
| 1,1'-Dioctadecyl-3,3,3',3'-tetramethylindotricarbocyanine iodide (DiR) | 750/780 | 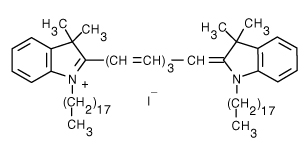 |
| 4-(4-(Dihexadecylamino)styryl)-N-methylpyridinium iodide (DiA) | 456/590 | 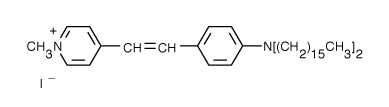 |
| 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2-1,3-benzoxadiazol-4-yl) (ammonium salt) (NBD on head group) | 460/535 | 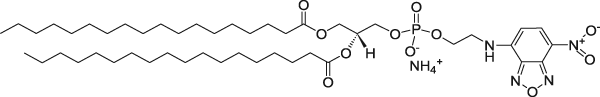 |
| 1-Palmitoyl-2-{12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]dodecanoyl}-sn-glycero-3-phosphocholine (NBD on fatty acid tail) | 460/534 | 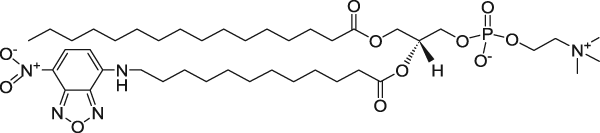 |
| 1,2-Dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (ammonium salt) (Rhodamine lipid) | 560/583 | 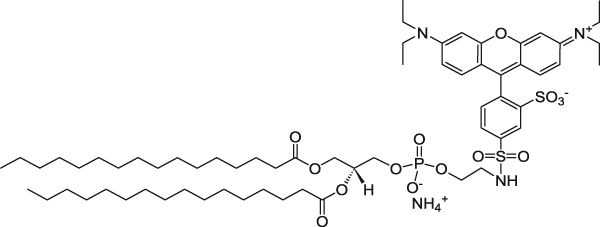 |
| 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine-N-(5-dimethylamino-1-naphthalenesulfonyl) (ammonium salt) (Dansyl lipid) | 336/513 | 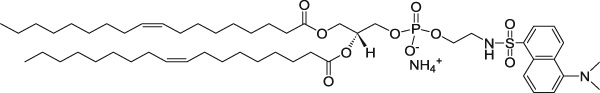 |
| 1,2-Dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(1-pyrenesulfonyl) (ammonium salt) (Pyrene lipid) | 351/379 | 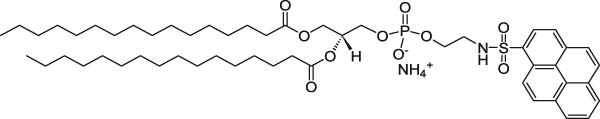 |
| Buffer and Liposome Size for Vial 1 | Specification |
|---|---|
| Buffer | Phosphate Buffered Saline |
| pH | 7.4 |
| Liposome Size | 100 nm |
| Vial 2 * | Specification |
|---|---|
| DSPE-PEG(2000)-NHS Lipid | This vial contains reactive DSPE-PEG(2000)-NHS lipid in powder form. This lipid is conjugated to a reactive protein, peptide or ligand containing amine and then mixed with non-reactive DSPE-PEG(2000) lipid in aqueous solution to form micelles. The PEGylated lipid micelles are incubated with preformed liposomes in vial 1 and PEG lipids will post-insert themselves into the liposomes. 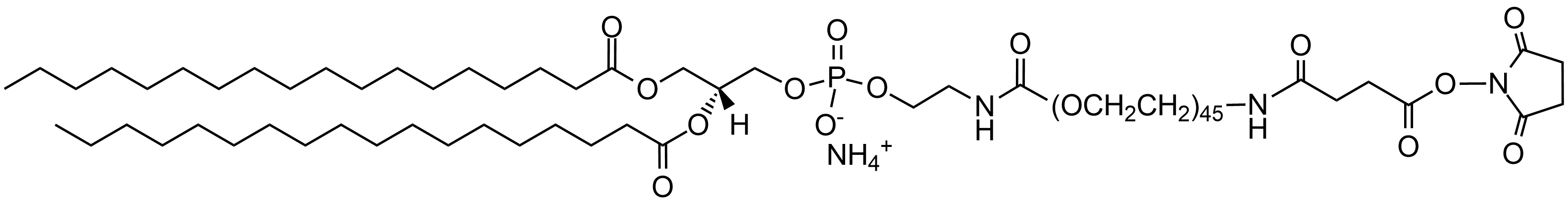 |
| * The amount of the powdered PEG(2000)-NHS lipid for the 2-ml kit is 1.34 mg and for the 5-ml kit is 3.34 mg. | |
| Vial 3 * | Specification |
|---|---|
| DSPE-PEG(2000) Lipid | This vial contains non-reactive DSPE-PEG(2000) lipid in powder form. This lipid in mixed with DSPE-PEG(2000)-NHS lipid which is already conjugated to a ligand (protein, peptide, etc.) in aqueous solution to form micelles. The PEGylated lipid micelles are incubated with preformed liposomes in vial 1 and PEG lipids will post-insert themselves into the liposomes.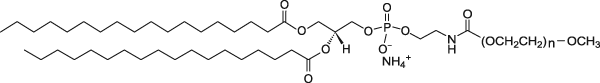 |
| * The amount of the powdered PEG(2000)-DSPE lipid for the 2-ml kit is 5 mg and for the 5-ml kit is 12.5 mg. | |
Conjugation Protocol (Post-insertion)
Materials and Equipment
The 3-vial post-insertion kit contains preformed liposomes (vial 1), non-reactive PEGylated lipid in powder form (vial 2) and DSPE-PEG(2000)-NHS lipid in powder form (vial 3). In order to use the post-insertion kit, you will need:
- Laboratory vortex mixer is recommended to have.
- Laboratory magnetic stirrer is needed for dialysis.
- Two small 10-ml round bottom flasks or two small glass vials.
- A rotary evaporator. We understand that many labs might not have a rotovap. Alternatively, you can use a nitrogen tank connected to a thin hose for creating a stream of nitrogen flow to dry the lipid and make a thin film.
- A small amount of a solvent such a chloroform or methylene chloride (you will only need a few milliliters).
- Phosphate buffered saline (PBS).
- A Sonicator. It is better to have a bath sonicator. If you do not, that is fine. You still can follow the protocol. You may also use a vortex instead of the sonicator for agitation of the solution as well.
- Float-A-Lyzer® with a proper MWCO that easily allows the cleanup of your liposome conjugated ligand from free and non-conjugated protein, peptide or antibody. You need to make sure that the MWCO is below 1,000,000 dalton. At 1,000,000 dalton the pore size on the dialysis membrane gets close to 100 nm and therefore, your liposomes can be dialyzed out. You cannot use dialysis cassettes blindly. Please understand the technique before using either spin column or dialysis cassette. If you do not use the correct MWCO, you can lose your entire prep. For this protocol, we recommend MWCO of 300,000 dalton.
Preparation Method
- Dissolve the content of vial 3 in 100 µl of chloroform or methylene chloride. Transfer the solution to a 10 ml round bottom flask. Dry the chloroform using a rotary evaporator or under a stream of nitrogen and make a dried lipid film.
- Add 100 µl of PBS buffer to the dried lipid film. It is preferred to sonicate the hydrated lipid film using a bath sonicator and sonicate the micelle solution for 5 minutes. If you do not have a bath sonicator then hydrate the dried lipid film with PBS for at least 1 hour and constantly rotate the solution in the round bottom flask using a rotavap (not connected to vacuum) or by hand to make sure that all the dried lipid on the wall of the round bottom flask will go to the solution and form micelles. Alternatively, you can use a vortex to agitate the solution. The goal is to have all the dried lipid on the wall of the round bottom glass to go the micelle solution. Cover the mouth of the round bottom flask with parafilm. Refrigerate the micelle solution of non-reactive PEG lipids until it is ready to be mixed with micelles formed in step 4.
- There is 1.34 mg (0.22 µmol) of DSPE-PEG-NHS in the 2-ml post-insertion kit. Dissolve the content of vial 2 in 100 µl of chloroform or methylene chloride. Transfer the solution to a 10 ml round bottom flask. Dry the chloroform using a rotary evaporator or under a stream of nitrogen.
- Add the water-soluble protein, peptide or ligand at 1:2 molar ratio of ligand to dried film of DSPE-PEG-NHS. Add 300 µl of water-soluble solution of protein of ligand in PBS (pH 7.4) to the dried lipid film. It is preferred to sonicate the hydrated lipid film using a bath sonicator and sonicate the micelle solution for 5 minutes. If you do not have a bath sonicator then hydrate the dried lipid film with PBS for at least 1 hour and constantly rotate the solution in the round bottom flask using a rotavap (not connected to vacuum) or by hand to make sure that all the dried lipid on the wall of the round bottom flask will go to the solution and form micelles. Alternatively, you can use a vortex to agitate the solution. The goal is to have all the dried lipid on the wall of the round bottom glass to go the micelle solution. The solution is incubated at room temperature for 6 hours and in refrigerator for 24 hours. The reaction is pH sensitive. Read the technical note below for more information.
- Mix the micelles in step 2 to the micelles in step 4. The total volume of micelles should be 400 µl.
- To conduct post-insertion, the micellar dispersion is then co-incubated with preformed plain liposomes at 60℃ for 30 min.
- Remove the non-conjugated protein, peptide or antibody from the immunoliposomes by dialysis. We prefer dialysis to size exclusion columns. Dialysis is a much slower process but there will be minimum loss of immunoliposomes after the prep is cleaned from non-conjugated protein/peptide/ligand. Spin columns are much faster; however, you can easily lose over 50% of the liposomes on the spin column. We recommend using Float-A-Lyzer® dialysis cassette from Spectrum Labs. You will need to choose a cassette with proper MWCO depending on the MW of your protein, peptide, antibody or antibody fragment. NOTE: If you decide to use a dialysis cassette, you will need to make sure that the MWCO is below 1,000,000 dalton. At 1,000,000 dalton, the pore size on the dialysis membrane gets close to 100 nm and therefore, your liposomes can be dialyzed out. You cannot use dialysis cassettes and spin columns blindly. They come in various sizes and you need to choose the correct size wisely. Dialyze the immunoliposome solution in 1 liter of PBS at pH 7.4 for 8 hours. Change the dialysis buffer with a fresh 1 liter of PBS and let is dialyze for another 8 hours. After this step, your cleaned up immunoliposome is ready to be used.
Liposome Particle Calculator
ImmunoFluor™ liposomes are unilamellar and sized to 100 nm. The molar concentration of liposome is 24.56 mM. By having liposome diameter (nm) and lipid concentration (µM), you can calculate the total number of the lipids in one liposome and the number of the liposomes in one milliliter of the liposome solution. To use the calculator click here.
Technical Notes
- Hydrolysis of DSPE-PEG-NHS in aqueous solutions competes with the primary amine reaction, resulting in the elimination of the NHS group, which can consequently decrease the coupling yield prior to the reaction with the protein/antibody. In order to minimize the impact of the hydrolysis of NHS ester, use a high concentration of protein/antibody to increase the efficiency of the cross-linking.
- The reaction of NHS esters with amines is strongly pH-dependent: at low pH, the amino group is protonated, and no modification takes place. At higher-than-optimal pH, hydrolysis of NHS ester is quick, and modification yield diminishes. The half-life of NHS esters at pH 7 and 8 is 4-5 hours and 1 hour, respectively. Whilst NHS esters have a half-life of only 10 minutes at pH 8.6. Therefore, to avoid the hydrolysis of NHS ester, DSPE-PEG-NHS lipid should be used immediately for conjugation to antibodies, proteins or peptides containing free amines. NHS ester reactions are conducted in common buffers at pH 7-8.
- Primary amine buffers such as Tris should NOT be used because they compete for reaction; however, in some procedures, it is useful to add Tris or glycine buffer at the end of a conjugation procedure to quench (stop) the reaction.
- If you are using a ligand or peptide that is hydrophobic, it is recommended to solubilize it in DMSO or DMF and then add the buffer to it. It is recommended not to use more than 5% volume of DMSO or DMF in the solution. DMF and DMSO are both compatible with liposomes and they are also miscible in water. Other organic solvent such as ethanol and chloroform are not compatible with liposomes and will cause the liposomes to lyse. If you end up using DMSO or DMF then after the conjugation reaction is done, you need to remove DMSO and DMF from the liposomes. In order to do that, you need to use a dialysis cassette that is made from REGENERATED CELLULOSE MEMBRANE. NOTE: Not all membranes are compatible with DMF and DMSO. We recommend using a Slide-A-Lyzer™ MINI Dialysis Device with MWCO of 2K made from regenerated cellulose membrane manufactured by ThermoFisher. After DMSO or DMF is removed you can use Float-A-Lyzer® dialysis device for the final step of cleaning up the prep.
- Liposomes should be kept at 4°C and NEVER be frozen.
Database
Direct link to the database page for easy navigation: Immunoliposomes Conjugation Database
Appearance
ImmunoFluor™-NHS (PEGylated) post-insertion kit comes in three vials: vial 1 formulation is colored and the color depends on the type of the fluorescent dye that is used. It contains nano size unilamellar liposomes which does not contain any reactive of non-reactive PEGylated lipid. Usually due to the small size of liposomes no settling will occur in the bottom of the vial. Vial 2 contains reactive DSPE-PEG(2000)-NHS lipid in white powder form. Vial 3 contains non-reactive DSPE-PEG(2000) lipid in white powder form.
Ordering/Shipping Information
- All liposome based formulations are shipped on blue ice at 4°C in insulated packages using overnight shipping or international express shipping.
- Liposomes should NEVER be frozen. Ice crystals that is formed in the lipid membrane can rupture the membrane, change the size of the liposomes and cause the encapsulated drug to leak out. Liposomes in liquid form should always be kept in the refrigerator.
- Clients who order from outside of the United States of America are responsible for their government import taxes and customs paperwork. Encapsula NanoSciences is NOT responsible for importation fees to countries outside of the United States of America.
- We strongly encourage the clients in Japan, Korea, Taiwan and China to order via a distributor. Tough customs clearance regulations in these countries will cause delay in custom clearance of these perishable formulations if ordered directly through us. Distributors can easily clear the packages from customs. To see the list of the distributors click here.
- Clients ordering from universities and research institutes in Australia should keep in mind that the liposome formulations are made from synthetic material and the formulations do not require a “permit to import quarantine material”. Liposomes are NOT biological products.
- If you would like your institute’s FedEx or DHL account to be charged for shipping, then please provide the account number at the time of ordering.
- Encapsula NanoSciences has no control over delays due to inclement weather or customs clearance delays. You will receive a FedEx or DHL tracking number once your order is confirmed. Contact FedEx or DHL in advance and make sure that the paperwork for customs is done on time. All subsequent shipping inquiries should be directed to Federal Express or DHL.
Storage and Shelf Life
Storage
ImmunoFluor™ products should always be stored at in the dark at 4°C, except when brought to room temperature for brief periods prior to animal dosing. DO NOT FREEZE. If the suspension is frozen, the encapsulated drug can be released from the liposomes thus limiting its effectiveness. In addition, the size of the liposomes will also change upon freezing and thawing.
Shelf Life
ImmunoFluor™-NHS is made on daily basis. The batch that is shipped is manufactured on the same day. It is advised to use the products within 2 months of the manufacturing date.











