m-Fluoroliposome®-DiR
SKU# CLD-8936
Description
Mannose receptor targeting by mannosylated liposomes has been demonstrated for a variety of mannosylated lipid conjugates in a variety of liposome morphologies and compositions in several different in vitro and in vivo models. There are several publications using a hydrophobic derivative of mannose (4-aminophenyl α-D-mannopyranoside) rather than using a mannosylated lipid in clodronate liposomes. This is mainly due to the high cost and complexity of synthesizing and conjugating mannose to lipid. 4-aminophenyl α-D-mannopyranoside is commercially available and far less expensive than synthesizing mannose conjugated lipid.
Why mannose? Mannose is one of the carbohydrate components of many bacterial and viral cell surfaces; therefore, the ever-efficient, highly redundant immune system has evolved multiple mechanisms for identifying pathogens based on mannose recognition. The animal and plant kingdoms likewise utilize carbohydrate recognition signaling mechanisms including mannose residues. Many publications evaluate other carbohydrates as targeting mechanisms for various cell types, however mannose targeting to phagocytes appears to be one of the more specific mechanisms identified to date. Mammalian cell surface identification molecules based on mannose binding, such as the ICAM family of leukocyte adhesion molecules, target the SIGN family of mannose receptors to accomplish self-recognition in vivo.
A well-known and cited study by Umezawa & Eto [1] demonstrates that liposomes containing aminophenyl mannoside were most efficiently incorporated into the mouse brain across the blood brain barrier. The radiolabeled liposomes bearing aminophenyl ????-D-mannopyranoside were maximally incorporated into the mouse brain after 48 hours, whereas in the spleen and liver, these radioactivities were maximum after 12 hours. The studies also showed that liposomes were most incorporated was glial cells rather than neuronal cell. The subcellular fractionation study indicates that mannose labeled liposomes are incorporated into lysosomes rich fraction both in liver and brain.
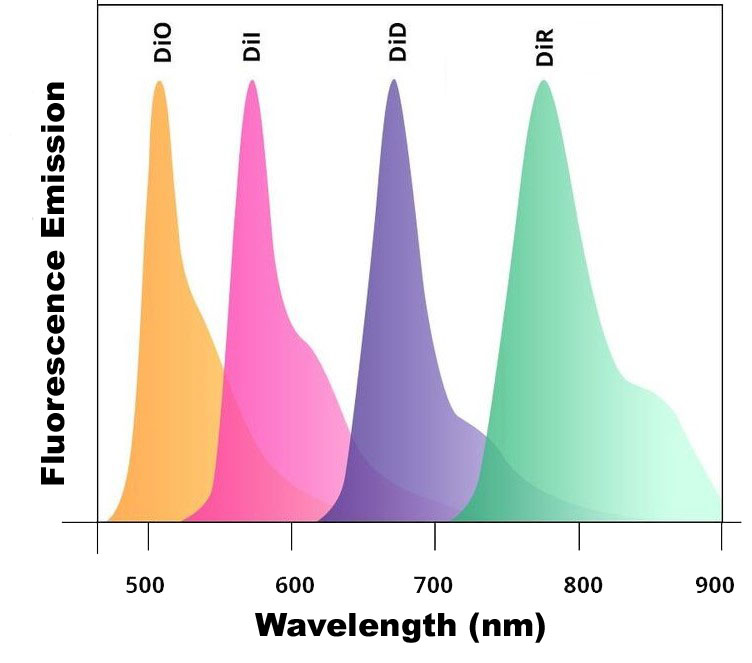
There are five mannosylated fluorescent control liposome products (m-Fluoroliposome®) for m-Clodrosome® (mannosylated clodronate liposomes). All five mannosylated fluorescent liposomes incorporate a lipophilic dye inside their membranes. They are insoluble in water; however, their fluorescence is easily detected when incorporated into membranes. DiI, DiO, DiD, DiR and DiA cover a wide range of excitation and emission wavelengths from 300s to 900s. DiI and DiO have fluorescence excitation and emission maxima separated by about 65 nm, facilitating two-color labeling. The emission spectrum of DiA is very broad, allowing it to be detected as green, orange, or even red fluorescence depending on the optical filter used. DiI, DiO, DiD and DiR belong to the dialkylcarbocyanines family of compounds. The spectral properties of the dialkylcarbocyanines are largely independent of the lengths of the alkyl chains but are instead determined by the heteroatoms in the terminal ring systems and the length of the connecting bridge. They have extremely high extinction coefficients, moderate fluorescence quantum yields, and short excited state lifetimes in lipid environments (~1 ns). The fluorescence spectrum of each dye is shown below.
You can choose the m-Fluoroliposome® based on the type of the fluorescent equipment and filters that you use in your lab. Mannosylated clodronate liposomes cannot be made fluorescent simply due to the potential for inaccurate and/or uninterpretable data being generated by labelled m-Clodrosome®. For more information, please refer to the technical note section.
Formulation Information
m-Fluoroliposome®-DiR
| Lipid Composition | Concentration (mg/ml) | Concentration (mM) | Molar Ratio Percentage |
|---|---|---|---|
| L-alpha-Phosphatidylcholine | 18.8 | 24.3 | 70 |
| Cholesterol | 4.2 | 10.9 | 30 |
| Total | 23 mg/ml | 35.1 mM | 100 |
| Mannosylation | Concentration |
|---|---|
| 4-Aminophenyl-alpha-D-mannopyranoside | 9.53 mol% |
| Fluorescent Dye | Excitation/Emission (nm) | Concentration (mg/ml) | Concentration (mM) |
|---|---|---|---|
1,1'-Dioctadecyl-3,3,3',3'-Tetramethylindotricarbocyanine Iodide (DiR)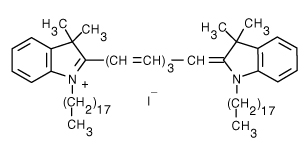 | 750/780 | 0.066 | 0.0651 |
| Buffer and Liposome Size | Specification |
|---|---|
| Buffer | Phosphate Buffered Saline |
| pH | 7.4 |
| Liposome Size | 1.5-2 µm |
Technical Notes
- The issue with fluorescent Clodrosome® has to do with the potential for inaccurate and/or uninterpretable data being generated by labelled Clodrosome®. When Clodrosome® induces macrophage apoptosis, the fluorescent lipid incorporated into the Clodrosome® is disrupted and metabolized in the phagolysosome will be dispersed among the residual apoptotic bodies which are subsequently phagocytosed by other macrophages. Therefore, fluorescent lipid may be detected in phagocytic cells which never phagocytosed Clodrosome® especially when FACS or fluoroscopy are utilized to detect fluorescent cells (FACS) or fluorescence levels in a tissue homogenate (fluoroscopy). Another potential artifact arises from fluorescent lipid remaining in the extracellular “garbage”, which has not yet been cleared by other phagocytes, generating a high background fluorescence. However, experienced confocal microscopist may be able to differentiate between the punctate fluorescence, resulting from fluorescent intact liposomes versus the more diffuse fluorescence characteristic of disrupted liposomes and some have successfully used fluorescent clodronate liposomes to visualize the cellular location of these liposomes by confocal microscopy in vivo [2]. A further complicating factor is that published data varies widely as to exactly when clodronate liposomes begin to induce apoptosis in macrophages. Mönkönnnen et al. show that macrophage death is measurable within the first hour after clodronate liposome treatment on RAW264 cells in vitro [3], while many others have reported no signs of macrophage apoptosis until several hours after treatment in vivo. The variability in the data is likely due to different liposomal formulations of clodronate as well as the vastly different experimental conditions. Therefore, as with most biological studies, especially those involving liposomes, the amount of time between treating the animal or cells with clodronate liposomes and the onset of apoptosis will need to be established in each experimental model. If the nature of the research demands that Clodrosome® be tracked rather than the control, Encapsula can provide DiI-labelled Clodrosome® upon request, and assuming that the Clodrosome® distribution can definitively be assessed prior to the onset of apoptosis, clear and valid data on the biodistribution of fluorescent Clodrosome® should be obtainable. Still, for most purposes, Fluoroliposome® (fluorescent control liposomes) will provide the required data with far fewer potential artifacts.
- When monitoring monocyte uptake in vivo in normal animals, the circulating monocytes may “disappear” or show reduced counts within the first 2 h post-injection due to margination of the monocytes post-liposome phagocytosis. These cells will re-enter the circulation within a few hours. Sunderkötter et al. demonstrate this phenomenon and discuss the behavior in detail. Also consider that circulating monocytes have a lifetime of about 24 h so labeled monocytes will be continually leaving the circulation, even in normal animals, due to aging of the monocytes [4].
- Liposomes may settle when left undisturbed for more than a few hours. Immediately prior to use, in order to ensure a homogeneous liposome suspension, slowly invert the vial several times until the suspension appears homogeneous by visual inspection. Vigorous or erratic shaking will not damage the liposomes but may induce foaming and bubble formation making it more difficult to accurately measure the desired dosage.
- If the personnel performing intravenous injections are not experienced in or familiar with, precautions for injecting larger volumes (~10% animal weight in ml), viscous liquids or particulate suspensions, consider having extra animals available in case serious injection-related adverse events occur. Dose control animals first to become familiar with large volume injections.
- When dosing intravenously, use standard precautions for dosing larger volumes to animals including the following: a) warm product to room temperature prior to dosing; b) ensure that all air bubbles are removed from the syringe prior to dosing. Intravenous injection of air bubbles may result in air emboli which can kill or seriously injure animals; c) inject product at a slow, steady rate of no more than 1 ml/min; d) decrease infusion rate if animals display any atypical reactions such as unusual agitation.
- Infusion-related adverse reactions usually involve the animal gasping for air or other seizure-like movements. Animals often recover with no apparent permanent injury, but any potential effects on experimental results must be assessed by the researcher.
- Liposomes should be kept at 4°C and NEVER be frozen.
Dosage
Appearance
m-Fluoroliposome®-DiR is a dark blue liquid suspension made of large micron size multilamellar liposomes. Due to their large size, some liposomes might settle to the bottom of the vial. If left sitting idle in the refrigerator, m-Fluoroliposome® will phase separate and form pellets in the bottom of the vial, leaving a clear solution on top. Therefore, the vial should be shaken to form a homogeneous solution prior to use.
Educational Videos
Ordering/Shipping Information
- All liposome based formulations are shipped on blue ice at 4°C in insulated packages using overnight shipping or international express shipping.
- Liposomes should NEVER be frozen. Ice crystals that form in the lipid membrane can rupture the membrane, change the size of the liposomes and cause the encapsulated drug to leak out. Liposomes in liquid form should always be kept in the refrigerator.
- Clients who order from outside of the United States of America are responsible for their government import taxes and customs paperwork. Encapsula NanoSciences is NOT responsible for importation fees to countries outside of the United States of America.
- We strongly encourage the clients in Japan, Korea, Taiwan and China to order via a distributor. Tough customs clearance regulations in these countries will cause delay in custom clearance of these perishable formulations if ordered directly through us. Distributors can easily clear the packages from customs. To see the list of the distributors click here.
- Clients ordering from universities and research institutes in Australia should keep in mind that the liposome formulations are made from synthetic material and the formulations do not require a “permit to import quarantine material”. Liposomes are NOT biological products.
- If you would like your institute’s FedEx or DHL account to be charged for shipping, then please provide the account number at the time of ordering.
- Encapsula NanoSciences has no control over delays due to inclement weather or customs clearance delays. You will receive a FedEx or DHL tracking number once your order is confirmed. Contact FedEx or DHL in advance and make sure that the paperwork for customs is done on time. All subsequent shipping inquiries should be directed to Federal Express or DHL.
Storage and Shelf Life
Storage
m-Fluoroliposome® products should always be stored at in the dark at 4°C, except when brought to room temperature for brief periods prior to animal dosing. DO NOT FREEZE. ENS is not responsible for results generated by frozen product.
Shelf Life
m-Fluoroliposome® products are made on daily basis. The batch that is shipped is manufactured on the same day. It is advised to use the products within 60 days of the manufacturing date.











