Immunodox®-DBCO (PEGylated) (Post-insertion)*
SKU# IMD-1012
Description
During the past five decades various types of chemistries have been used for conjugation of molecules such as antibodies, peptides, proteins or other reactive ligands to the surface of the liposomes. In general the conjugation can be achieved through the N-terminus, the C-terminus or the available sulfur (eg. Fab’ fraction or thiolated antibodies). Not all chemistries have the same yield and efficiency of conjugation and often reproducing biocompatible batches can be a challenge.
Copper-free click chemistry is a fairly new chemistry that has been commercialized during the past few years. More and more click chemistry based reagents are becoming available commercially which makes the formulation development much easier for scientists. By far, click chemistry is the most efficient and easiest conjugation chemistry available for coupling of antibodies and other reactive ligands to the surface of the liposomes. The conjugation chemistry is based on the reaction of the dibenzocyclooctyne (DBCO) reagent with an azide linker to form a stable triazole. DBCO moiety can be on the antibody and azide moiety can be on liposomes and vice versa.
Post-insertion method is used for the formation of targeted liposomes using various whole antibodies or their fragments, peptides and other ligands. In this method, the antibody or peptide is first coupled to polyethylene micelles, and then incubated with preformed liposomes, which could have already been drug-loaded. Therefore, post-insertion can be used to design and form targeted liposomes by inserting various antibodies and peptides into the liposomes containing various drugs. Since separate conditions are applied for the drug loading and ligand conjugation, the processes can be optimized and the preparation complexities are minimized. Therefore, post-insertion, is a simple, flexible and effective approach for preparing tailored liposomes.
There are many commercialized reagent that can be used for azide modification of proteins, peptide and antibodies. To see the list of commercialized reagents for azide modification see here.
DBCO is a hydrophobic moiety and can bury itself into the lipid bilayers of the liposomes. The yield of the conjugation significantly increases if ligand-conjugated DBCO is post-inserted into the liposomes rather than using liposomes containing DBCO on the surface and conjugating the ligand conjugated azide to the surface of the reactive liposomes.
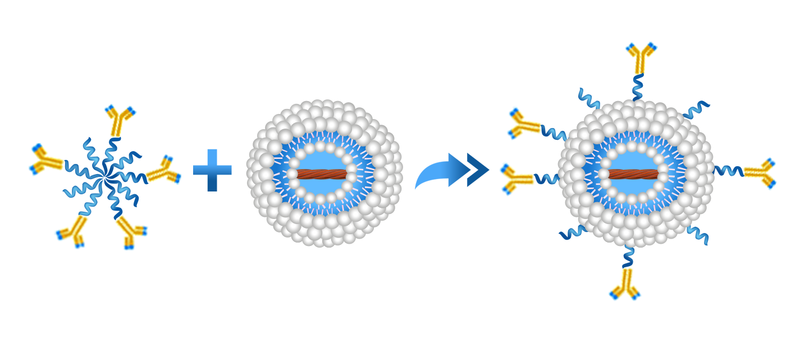
Formulation Information
Immunodox®-DBCO (PEGylated) (Post-insertion)
| Post Insertion Kit (3 Vials) | Specification |
|---|---|
| Vial 1 | Preformed Doxorubicin Liposomes composed of HSPC and Cholesterol (60:40 molar ratio) |
| Vial 2 | DSPE-PEG(2000)-DBCO lipid (reactive PEGylated lipid) in powder form |
| Vial 3 | DSPE-PEG(2000) lipid (non-reactive PEGylated lipid) in powder form |
| Lipid Composition for Vial 1* | Concentration (mg/ml) | Concentration (mM) | Molar Ratio Percentage |
|---|---|---|---|
| Hydrogenated Soy PC | 11.5 | 14.66 | 60 |
| Cholesterol | 3.83 | 9.9 | 40 |
| Total | 15.33 mg/ml | 24.56 mM | 100 |
| * For the 5-ml kit, the volume of vial 1 is 4 ml. 1 ml of micelle solution that are formed using vials 2 and 3 will be added to this vial to make the final volume of 5 ml in the final product. For the 2-ml kit, the volume of vial 1 is 1.6 ml. 0.4 ml of micelle solution that is formed using vials 2 and 3 will be added to this vial to make the final volume of 2 ml in the final product. | |||
| Buffers, Liposome Size and Encapsulated Drug Concentration for Vial 1 | Specification |
|---|---|
| Inside Buffer | Ammonium Sulfate |
| Outside Buffer | Phosphate Buffered Saline |
| pH | 7.4 |
| Liposome Size | 100 nm |
| Encapsulated Doxorubicin | 2.5 mg/ml (4.31 mM) |
| Vial 2 | Specification |
|---|---|
| DSPE-PEG(2000)-DBCO Lipid | This vial contains reactive DSPE-PEG(2000)-DBCO lipid in powder form. This lipid is conjugated to a reactive protein, peptide or ligand containing azide and then mixed with non-reactive DSPE-PEG(2000) lipid in aqueous solution to form micelles. The PEGylated lipid micelles are incubated with preformed liposomes in Vial 1 and PEG lipids will post-insert themselves into the liposomes. 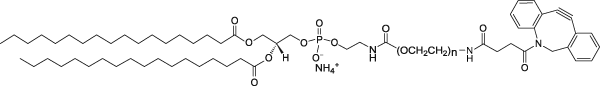 |
| Vial 3 | Specification |
|---|---|
| DSPE-PEG(2000) Lipid | This vial contains non-reactive DSPE-PEG(2000) lipid in powder form. This lipid in mixed with DSPE-PEG(2000)-DBCO lipid which is already conjugated to a ligand (protein, peptide, etc) in aqueous solution to form micelles. The PEGylated lipid micelles are incubated with preformed liposomes in Vial 1 and PEG lipids will post-insert themselves into the liposomes.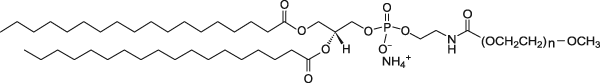 |
Conjugation Protocol (Post-insertion)
Materials and Equipment
The 3-vial post-insertion kit contains preformed liposomes (Vial 1), non-reactive PEGylated lipid solubilized in a solvent such as chloroform or methylene Chloride (Vial 2), and DSPE-PEG(2000)-DBCO lipid solubilized in a solvent such as chloroform or methylene Chloride (Vial 3). To use the post-insertion kit, you will need:
- Two small 10 ml round bottom flasks or two small glass vials
- A rotary evaporator. We understand that many labs might not have a rotovap. Alternatively, you can use a nitrogen or argon tank connected to a thin hose to create a stream of gas flow to dry the lipid and make a thin film.
- Dimethylformamide (DMF) if your ligend is liposoluble and soluble in DMF. If you use a water-soluble ligand, antibody, or peptide, then you do not need to use DMF.
- Phosphate-buffered saline (PBS)
- A Sonicator. It is better to have a bath sonicator. If you do not, that is fine. You can still follow the protocol. You may also use a vortex instead of the sonicator for agitation of the solution.
Preparation Method
- The post insertion kits come in two sizes: 2 ml and 5 ml. Transfer the solvent-solubilized non-reactive PEG lipid in vial 2 to a 10 ml round bottom flask or vial. Dry the chloroform using a rotary evaporator or under a stream of nitrogen or argon.
- For the 2 ml kit, add 100 µl of PBS buffer to the dried lipid film. For the 5 ml kit, the added buffer is 250 µl. It is preferred to sonicate the hydrated lipid film using a bath sonicator and sonicate the micelle solution for 5 minutes. If you do not have a bath sonicator, then hydrate the dried lipid film with PBS for at least 1 hour and constantly rotate the solution in the round bottom flask using a rotavap (not connected to a vacuum) or by hand to make sure that all the dried lipid on the wall of the round bottom flask will go to the solution and form micelles. Alternatively, you can use a vortex to agitate the solution. The goal is to have all the dried lipids on the wall of the round bottom glass go into the micelle solution. Cover the mouth of the round bottom flask with parafilm. Refrigerate the micelle solution of non-reactive PEG lipids until it is ready to be mixed with micelles formed in step 4.
- The 2 ml kit contains 1.34 mg (0.48 µmol) of reactive DSPE-PEG2000-DBCO lipid. The 5 ml kit contains 3.35 mg (1.2 µmol) of reactive DSPE-PEG2000-DBCO lipid. Co-dissolve 1 mol equivalent of the Azide-labeled peptide, antibody or ligand along with 2.5 mol equivalents of the reactive lipid (DSPE-PEG-DBCO) in PBS for water-soluble ligand, peptide or antibody, or DMF for liposoluble ligand. It is recommended to keep the volume of the peptide/lipid mixture in PBS or DMF solution to 200 µl for the 2 ml kit and 500 µl for the 5 ml kit. Incubate the solution at room temperature for 6 hours, then refrigerate for 24 hours.
- If you have used DMF to solubilize your liposoluble ligand then you need to clean up your micelles from DMF, Add 200 µl of PBS for the 2 ml kit and 500 µl of PBS for 5 ml kit to DMF peptide/lipid mixture and transfer the solution into a dialysis cassette that is made of REGENERATED CELLULOSE MEMBRANE. We very strongly recommend using Thermo Scientific Slide-A-Lyzer 2K MWCO Mini Dialysis Devices. Dialyze the solution against 1 liter of PBS buffer for 8 hours and change the buffer at least once more. Only use this step in you have DMF .
- The micelles obtained from step 2 and 4 are mixed. Total volume of the 2 mixed micelles for 2 ml kit is 400 µl and for 5 ml kit is 1000 µl.
- Incubate the mixed micelles with preformed liposomes (Vial 1) at 60 °C for 1 hour.
- Remove the non-conjugated protein, peptide or antibody from the immunoliposomes by dialysis. We prefer dialysis to size exclusion columns. Dialysis is a much slower process but there will be minimum loss of immunoliposomes after the prep is cleaned from non-conjugated protein/peptide/ligand. Spin columns are much faster; however, you can easily lose over 50% of the liposomes on the spin column. We recommend using Float-A-Lyzer® dialysis cassette from Spectrum Labs. You will need to choose a cassette with proper MWCO depending on the MW of your protein, peptide, antibody or antibody fragment. NOTE: If you decide to use a dialysis cassette, you will need to make sure that the MWCO is below 1,000,000 dalton. At 1,000,000 dalton, the pore size on the dialysis membrane gets close to 100 nm and therefore, your liposomes can be dialyzed out. You cannot use dialysis cassettes and spin columns blindly. They come in various sizes and you need to choose the correct size wisely. Dialyze the immunoliposome solution in 1 liter of PBS at pH 7.4 for 8 hours. Change the dialysis buffer with a fresh 1 liter of PBS and let is dialyze for another 8 hours. After this step, your cleaned up immunoliposome is ready to be used.
Liposome Particle Calculator
Immunodox® liposomes are unilamellar and sized to 100 nm. The molar concentration of liposome is 21.58 mM. By having liposome diameter (nm) and lipid concentration (µM), you can calculate the total number of the lipids in one liposome and the number of the liposomes in one milliliter of the liposome solution. To use the calculator click here.
Technical Notes
- Before starting the conjugation process please make sure to avoid buffers that contain azides, which can react with DBCO.
- Doxorubicin is a fluorescent molecule with λex 470 nm; λex 585 nm. If you are using a fluorescent tag on you antibody or ligand, then you need to make sure that they won’t interfere with each other.
- Reactions of DBCO and azides are more efficient at high concentrations and temperatures (i.e., up to 37 °C). In order to avoid denaturation of proteins, peptides and antibodies it is recommended to incubate molecules with liposomes at room temperature followed by refrigeration (see step 1).
- Typical reaction times are less than 12 h, however, incubating for longer can improve efficiency.
- Spin columns can be used for the immunoliposome separation, and they are very fast method for purification. However, a large quantity of the samples is lost on the column. Dialysis is a slower process with minimal sample loss and therefore, we recommend dialysis over spin columns.
- If you are using a ligand or peptide that is hydrophobic then it is recommended to solubilize it in DMSO or DMF and then add the buffer to it. It is recommended not to use more than 5% volume of DMSO or DMF in the solution. DMF and DMSO are both compatible with liposomes and they are also miscible in water. Other organic solvent such as ethanol and chloroform are not compatible with liposomes and will cause the liposomes to lyse. If you end up using DMSO or DMF then after the conjugation reaction is done, you need to remove DMSO and DMF from the liposomes. In order to do that you need to use a dialysis cassette that is made from REGENERATED CELLULOSE MEMBRANE. NOTE: Not all membranes are compatible with DMF and DMSO. We recommend using a Slide-A-Lyzer™ MINI Dialysis Device with MWCO of 2K made from regenerated cellulose membrane manufactured by ThermoFisher. After DMSO or DMF is removed, you can use Float-A-Lyzer® dialysis device for the final step of cleaning up the prep.
- Liposomes should be kept at 4°C and NEVER be frozen.
Database
Direct link to the database page for easy navigation: Immunoliposomes Conjugation Database
Appearance
Immunodox®-DBCO is a red translucent liquid made of nano size unilamellar liposomes. Usually due to the small size of liposomes no settling will occur in the bottom of the vial. The liposomes are packaged in an amber vial.
Educational Video
Ordering/Shipping Information
- All liposome based formulations are shipped on blue ice at 4°C in insulated packages using overnight shipping or international express shipping.
- Liposomes should NEVER be frozen. Ice crystals that form in the lipid membrane can rupture the membrane, change the size of the liposomes and cause the encapsulated drug to leak out. Liposomes in liquid form should always be kept in the refrigerator.
- Clients who order from outside of the United States of America are responsible for their government import taxes and customs paperwork. Encapsula NanoSciences is NOT responsible for importation fees to countries outside of the United States of America.
- We strongly encourage the clients in Japan, Korea, Taiwan and China to order via a distributor. Tough customs clearance regulations in these countries will cause delay in custom clearance of these perishable formulations if ordered directly through us. Distributors can easily clear the packages from customs. To see the list of the distributors click here.
- Clients ordering from universities and research institutes in Australia should keep in mind that the liposome formulations are made from synthetic material and the formulations do not require a “permit to import quarantine material”. Liposomes are NOT biological products.
- If you would like your institute’s FedEx or DHL account to be charged for shipping, then please provide the account number at the time of ordering.
- Encapsula NanoSciences has no control over delays due to inclement weather or customs clearance delays. You will receive a FedEx or DHL tracking number once your order is confirmed. Contact FedEx or DHL in advance and make sure that the paperwork for customs is done on time. All subsequent shipping inquiries should be directed to Federal Express or DHL.
Storage and Shelf Life
Storage
Immunodox® products should always be stored at in the dark at 4°C, except when brought to room temperature for brief periods prior to animal dosing. DO NOT FREEZE. If the suspension is frozen, the encapsulated drug can be released from the liposomes thus limiting its effectiveness. In addition, the size of the liposomes will also change upon freezing and thawing.
Shelf Life
Immunodox®-DBCO is made on daily basis. The batch that is shipped is manufactured on the same day. It is advised to use the products within 4 months of the manufacturing date.











