Intrabursal (Ovary) Administration of Clodronate Liposomes
Only one paper has been published on the ovarian intrabursal administration of clodronate liposomes [1] to date. The authors provided a good review of their injection method, however Connolly, et. al. [2] and Codero, et. al. [4] presented a very detailed description of the technique for sucessful injection into the intrabursal space around the ovary [2].
Van der Hoek, et. al. only accomplished about 38% (day -3) and 33% (day -1) depletion in the thecal macrophages and 16% (day -3) and 10% (day -1) in the stromal macrophages, at best. Moreover the depletion was specific to F4/80+ macrophages in the stroma while all phenotypes were affected in the theca [1]. The proportion of cells depleted between days -3 and -1 (see experimental timeline below) remained similar, however the total number (controls) of Ia (MHC II)+ and F4/80+ macrophages appeared to increase from day -3 to day -1. When we extrapolated the graphical data from the publication, the increase approached significance in these groups, but not in the F4/11+ subpopulation. The variation in depletion of the macrophage phenotypes and the tissue distribution requires further investigation.
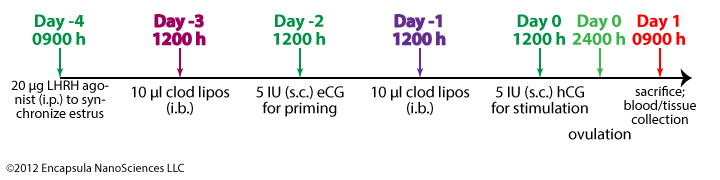
While we have no expertise in the structure and function of the different tissues of the ovaries, we believe that van der Hoek, et. al. have made the assumption that liposomes freely migrate across endothelial barriers into tissues. Even when the endothelial cell barrier allows for liposome entrance into a tissue, liposomes do not freely migrate throughout the tissue as many small water-soluble molecules do [5]. Liposomes must be carried by fluid flow (as in the bloodstream) or their contents can be delivered by monocytes/macrophages which have phagocytosed the liposomes (see trojan horse mechanism on the intravenous administration page). Although van der Hoek, et. al. report that fluorescent liposomes entered the thecal and stromal tissues of the ovaries after intrabursal administration, it is likely that this entry was mediated by macrophages removing the liposomes from the bursal surface of the ovarian endothelium. This is supported by the observation that even when the select macrophage populations appear to increase in total number (between days -3 and -1), the proportion of macrophages that were depleted do not change significantly. It appeared that a portion of the macrophages could access the liposomes and that when the total number of macrophages increased, that portion also increased and was able to access liposomes. If the total number of macrophages increased, but the same number of macrophages were depleted as when fewer macrophages were present, we might have considered that the population of “new” macrophages did not have access to the liposomes.
Due to the invasive nature of the intrabursal injection procedure, we assume that it would not be possible to deliver multiple doses of liposomes (i.e. daily doses) to the ovaries. Additionally there appears to be a normal increase in ovarian tissue macrophages as the estrus cycle approaches ovulation [3]. We assume that this increase is a result of monocytes being recruited from the bloodstream, thus monocyte depletion by intravenous injection of clodronate liposomes earlier in the cycle and continuing through the experimental period should limit the number of macrophages in the ovaries. An additional intrabursal dose of clodronate liposomes should further reduce the number of resident macrophages in the ovaries. While we realize that systemic depletion may complicate the interpretation of resulting data, it may provide an answer to the question of whether or not a complete macrophage depletion would prevent ovulation. Furthermore, depletion of circulating monocytes may reveal more information regarding the behavior of the different macrophage phenotypes in the ovaries.
References
- van der Hoek KH, Maddocks S, Woodhouse CM, van Rooijen N, Robertson SA, Norman RJ. Intrabursal Injection of Clodronate Liposomes Causes Macrophage Depletion and Inhibits Ovulation in the Mouse Ovary. Biology of Reproduction. 2000 Apr 1;62(4):1059 –1066.
- Connolly DC, Hensley HH. Xenograft and Transgenic Mouse Models of Epithelial Ovarian Cancer and Non-Invasive Imaging Modalities to Monitor Ovarian Tumor Growth In Situ: Applications in Evaluating Novel Therapeutic Agents. Current Protocols in Pharmacology [Internet]. John Wiley & Sons, Inc.; 2001 [cited 2012 Jul 30]. Available from: https://onlinelibrary.wiley.com/doi/10.1002/0471141755.ph1412s45/abstract
- Wu R, van der Hoek KH, Ryan NK, Norman RJ, Robker RL. Macrophage contributions to ovarian function. Human Reproduction Update. 2004 Mar 1;10(2):119 –133.
-
Cordero AB, Kwon Y, Hua X, Godwin AK. In vivo Imaging and Therapeutic Treatments in an Orthotopic Mouse Model of Ovarian Cancer. Journal of Visualized Experiments [Internet]. 2010 Aug 17 [cited 2012 Aug 1];(42). Available from: https://www.jove.com/video/2125/in-vivo-imaging-therapeutic-treatments-an-orthotopic-mouse-model
-
Ishida O, Maruyama K, Sasaki K, Iwatsuru M. Size-dependent extravasation and interstitial localization of polyethyleneglycol liposomes in solid tumor-bearing mice.International Journal of Pharmaceutics. 1999 Nov 10;190(1):49–56.











