Liposome Stability
The difficulty in using liposomes in a food or nutraceutical formulation centers on the stability of the phospholipids at room temperature. Chemical degradation such as hydrolysis and/or oxidation, aggregation, and fusion often occur overtime.
Hydrolysis
Hydrolysis is a natural process. Both saturated and unsaturated lipids will hydrolyze over time. The hydrolysis of a single molecule of phosphatidylcholine provides the following five hydrolysis products: 1-Acyl-Lyso-PC, 2-Acyl-Lyso-PC, free fatty acids, and glycerophosphorylcholine. In Figure 1, the hydrolysis of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) as the most natural occurring lipid is shown.
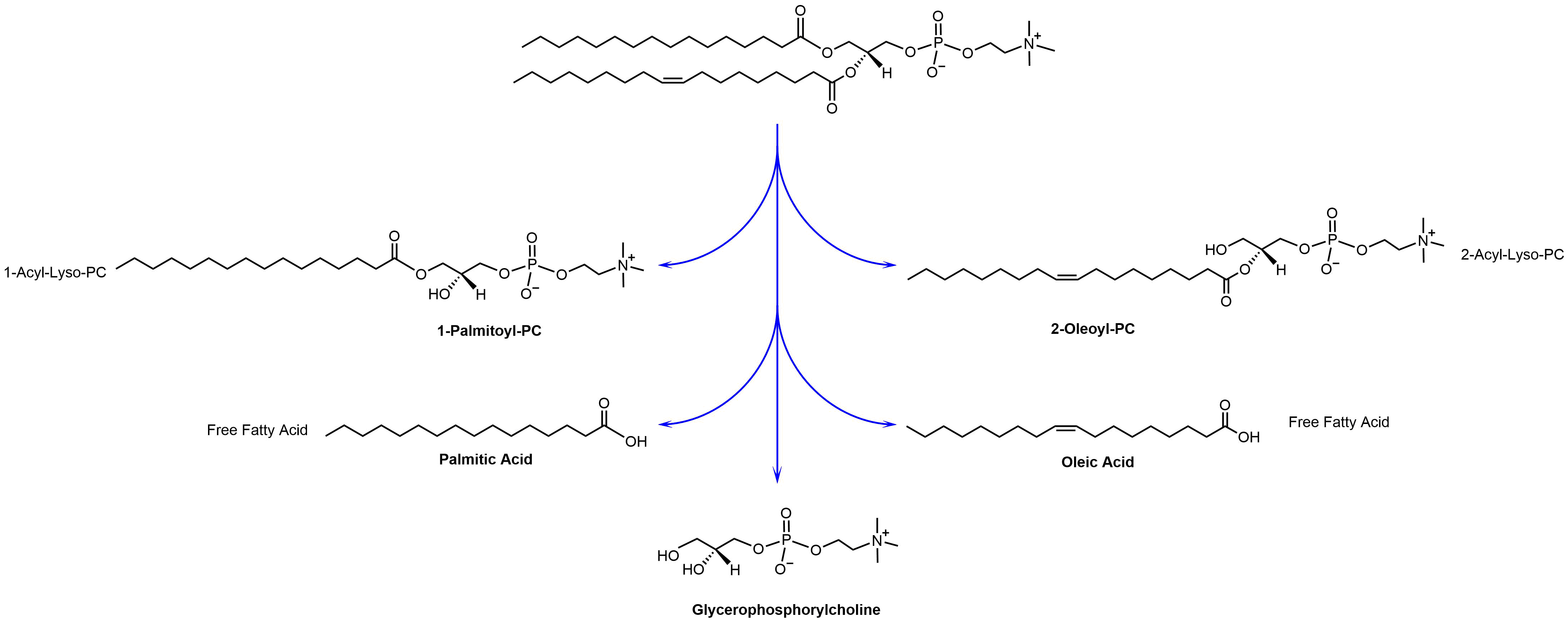
The formation of lyso-PC is one of the main reasons for chemical and physical instability of liposomes. Lyso-PC has very strong detergent properties. Lyso-PC got its name due to its ability to lyse red blood cells. Lyso-PC can exchange into and out of liposomal membrane and it can destabilize bilayers resulting in leakage of encapsulated aqueous contents. Lyso-PC can also stabilize non-bilayer forming lipid into bilayers and induce aggregation and/or fusion in liposome formulations.
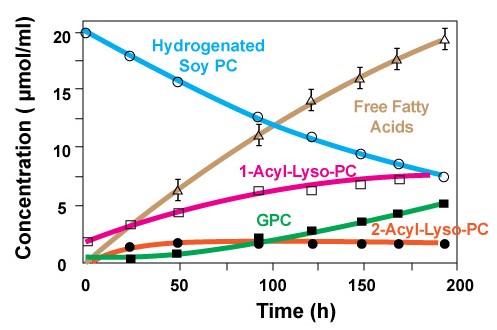
As it is shown in figure 2, over an 8 day span the concentration of hydrogenated Soy PC disappears as the products of hydrolysis increase. Keep in mind that in this study extreme conditions such as high temperature and low pH is used [1].
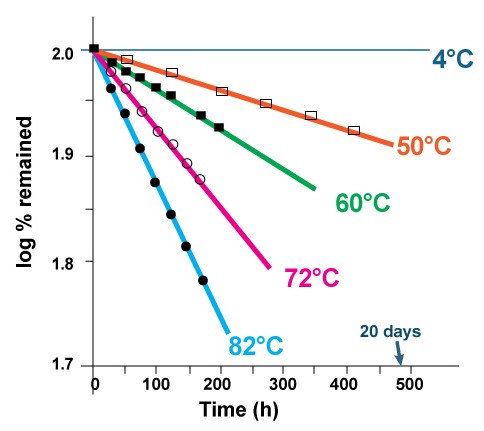
There are several ways to decrease the rate of hydrolysis and formation of lyso-PC and therefore to increase the shelf-life of the liposomes. The rate of hydrolysis is temperature dependent. As it was shown in figure 3, over 40% of the lipid degrades after 8 days, when the liposome solution is kept at 82°C. There is almost no lipid degradation when the same liposome solution is kept at 4°C in refrigerator [2].
Buffer and pH
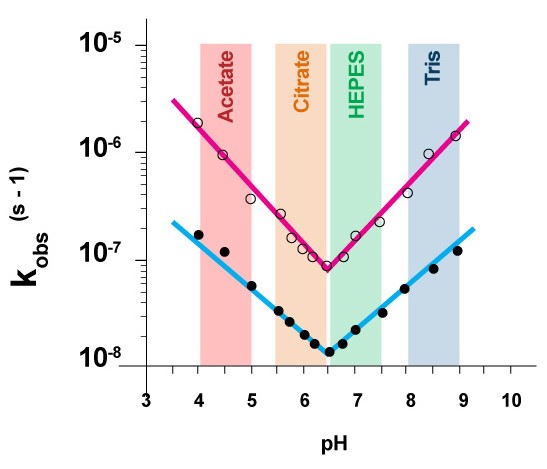
Hydrolysis reaction produces free protons (H+). Phospholipid acid hydrolysis is autocatalytic. Without buffer hydrolysis accelerates and therefore the liposome formulation must be buffered. In figure 4, first order rate constant k’obs at each pH value is calculated from lipid degradation data at 72 °C in various citrate buffer concentrations and then extrapolated to zero concentration of citrate. The experiment shows that in order to maximize the shelf life of the liposomes, high concentration of buffer should be avoided, and minimum effective concentration of buffer should be used.
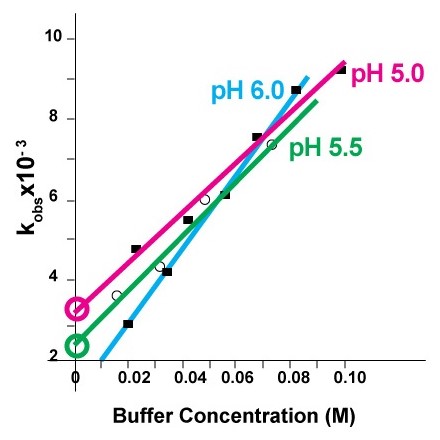
Figure 5 shows that at pH 6.5 lipids have the lowest rate of hydrolysis regardless of the temperature of their storage [3].
Oxidation of Unsaturated Phospholipids
The oxidation of the double bond in unsaturated phospholipid can be an issue. However, in turns out to not be a major issue if the formulation is composed of mono saturated phospholipids of high purity. Oxidation of lipids only becomes problematic when the formulation is composed of poly unsaturated phospholipids. Oxidation of phospholipids can be significantly decreased when the buffers are degassed and purged with nitrogen or argon.
Saturated Ether Lipids
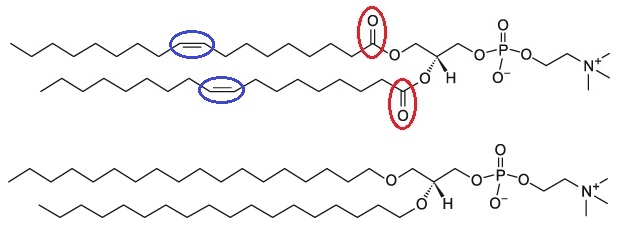
As it was discussed above, there are two reasons for instability of liposome formulation; hydrolysis and to a less extent oxidation. It is possible to use phospholipids that can neither be hydrolyzed nor oxidized. This class of phospholipids is called saturated ether lipids (see figure 6). These lipids are synthetic and very expensive and have limited research application currently. Due to the high cost of the lipid, they cannot be used in food and nutraceutical industry [4].
References
- Grit M, Crommelin D. Chemical stability of liposomes: implications for their physical stability. Chemistry and Physics of Lipids. 1993;64(1-3):3-18.
- Grit M, Desmidt J, Struijke A, Crommelin D. Hydrolysis of phosphatidylcholine in aqueous liposome dispersions. International Journal of Pharmaceutics. 1989;50(1):1-6.
- Grit M, Underberg WJM, Crommelin DJA. Hydrolysis of saturated soybean phosphatidylcholine in aqueous liposome dispersions. J. Pharm. Sci. 1993;82(4):362-366
- H.K. Mangold (1983), Ether lipids: Biochemical and Biomedical Aspects, Academic Press











