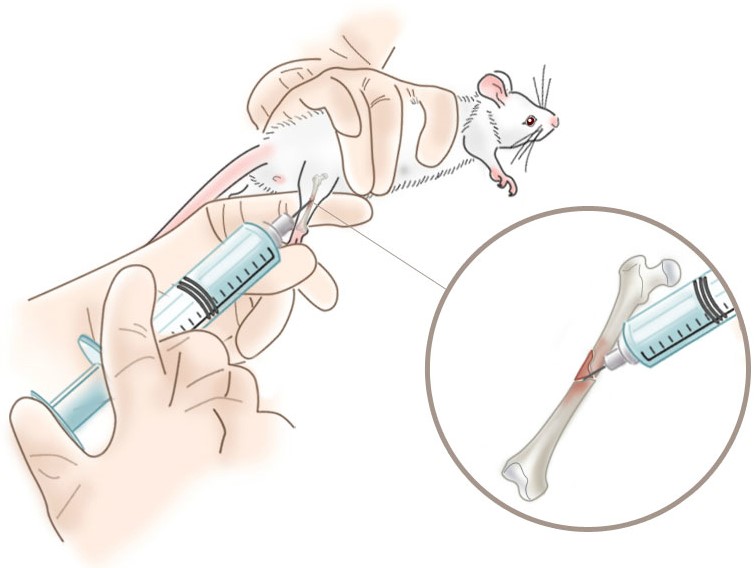
Intradefect Administration of Clodronate Liposomes
We have identified only one reference to intradefect administration of clodronate liposomes into injured bone, however other systemic routes of administration have been used to study the effects of osteal macrophage (osteomac) depletion on bone healing and remodeling as discussed on those pages and was employed in this study. In addition to injecting clodronate liposomes directly into the surgically-introduced “hole” in the mouse tibia immediately after the bone was injured, the liposomes were also dosed intraperitoneally (i.p.) daily for the 9 days between the bone injury and data collection. With this dosing regimen, some inflammatory macrophages would be recruited to the site of the injury releasing a limited concentration of inflammatory mediators prior to their death.
The authors did not investigate the possibility that pre-treatment of the mice 18-24 hours before the surgical intervention may have changed the outcome because this would have completely prevented the inflammatory phase (days 1-3) of bone healing. Likewise, withholding clodronate liposome treatment until after the inflammatory phase may have altered the results. Perhaps most importantly, the direct application of clodronate liposomes to the bone injury must have resulted in some level of free clodronate being released into the bone. Since the bone is not subject to a substantial rate of fluid exchange as in the bloodstream, the free clodronate may have remained inside the bone for some time, plus the death of local macrophages which had phagocytosed clodronate liposomes will likely release more free clodronate for the first 18 hours or so post-clodronate liposome injection into the bone.
Osteoclasts are the target cells for systemically dosed free clodronate, therefore we believe that local osteoclasts may have also been depleted by the local clodronate liposome administration. While osteoclast depletion may or may not be a factor in this model, a free clodronate control group would have clarified whether or not osteoclast depletion could have affected the results. The authors were quite diligent by including a control liposome group, but we must emphasize that free clodronate can effect local cells in models that involve local administration of clodronate liposomes. It is only in situations, such as the bloodstream, where clodronate released from either the liposomes or dying macrophages is quickly diluted and removed from the extracellular space, that free clodronate can be ignored as a potential effector on phagocytic cells. The page on Intrapulmonary administration of clodronate liposomes discusses this in detail. Liposomally-encapsulated clodronate when phagocytosed by cells is significantly more effective in killing macrophages, but free clodronate can be toxic to phagocytic cells—osteoclasts in particular.
With these factors in mind, we recommend thorough consideration of the potential effects free clodronate after localized administration of clodronate liposomes including free clodronate control groups in models where free clodronate may not be effectively diluted and removed immediately after release from the liposomes or dying macrophages.
A more thorough discussion of liposomal clodronate effects on the bone marrow is presented on the Intravenous administration page.











