ImmunoFluor™-Biotin (Non-PEGylated)
Description
Biotinylated liposomes can be conjugated non-covalently with (strept)avidin through either direct interaction with the protein/antibody conjugated to (strept)avidin or by coupling with other biotinylated proteins using (strept)avidin as a bridging molecule. Both avidin and (strept)avidin form strong non-covalent bond with biotin. The high resistance to breakdown makes them very useful in bioconjugate chemistry. However, (strept)avidin has replaced avidin in most bioconjugation applications due to its enhanced properties. NeutrAvidin (ThermoFisher) is a modified avidin without negative properties. It performs much better than original avidin and sometimes (strept)avidin.
In order to exploit the high-affinity interaction of biotin with (strept)avidin, a two-step “sandwich” protocol (Method A) has been developed for the preparation of targeted immunoliposomes. In this methodology, (strept)avidin is first attached to biotinylated liposomes, then a biotin-modified protein/antibody is introduced into the biotinylated (strept)avidin-labeled liposomes. This non-covalent approach is rapid, extremely versatile and applicable to numerous targeting ligands of interest with respect to in vitro and in vivo applications. Alternatively, instead of forming a (strept)avidin bridge, (strept)avidin molecule can also be covalently conjugated to antibody or ligand (Method B) and non-covalently bound to liposomes containing biotin on surface in order to form immunoliposomes.
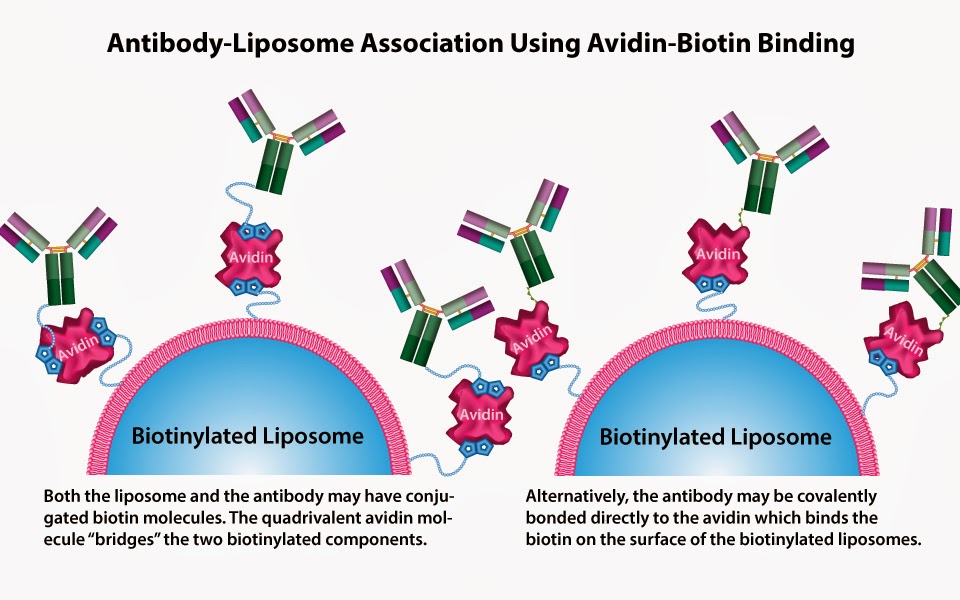
ImmunoFluor™-Biotin is a Non-PEGylated product. For other reactive (PEGylated and non-PEGyalated products) biotinylated liposomes and also ImmunoFluor™ products suitable for other types conjugation methods see here.
Formulation Information
ImmunoFluor™-Biotin (Non-PEGylated)
| Lipid Composition | Concentration (mg/ml) | Concentration (mM) | Molar Ratio Percentage |
|---|---|---|---|
| L-alpha-Phosphatidylcholine | ~12 | ~15.5 | ~69 |
| Cholesterol | 2.6 | 6.73 | 30 |
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(biotinyl) (sodium salt)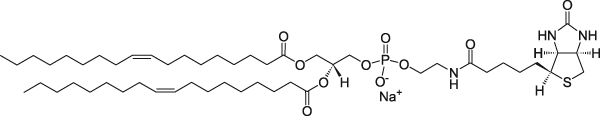 | 0.22 | 0.22 | 1 |
| Fluorescent Lipid (see below) | Varies based on the dye | Varies based on the dye | Varies based on the dye |
| Total | ~14.82 mg/ml | ~22.45 mM | 100 |
| Fluorescent Dye | Excitation/Emission (nm) | Molecular Structure |
|---|---|---|
| 1,1'-Dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI) | 549/565 | 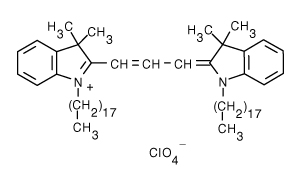 |
| 3,3'-Dilinoleyloxacarbocyanine perchlorate (DiO) | 484/501 | 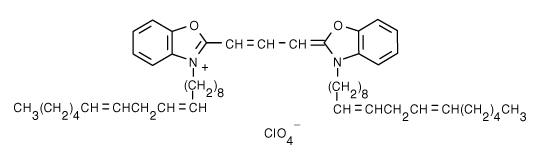 |
| 1,1'-Dioctadecyl-3,3,3',3'-tetramethylindodicarbocyanine, 4-chlorobenzenesulfonate salt (DiD) | 644/665 | 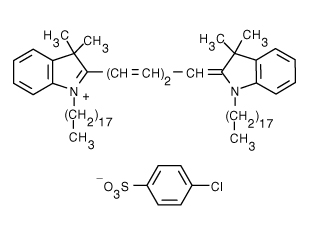 |
| 1,1'-Dioctadecyl-3,3,3',3'-tetramethylindotricarbocyanine iodide (DiR) | 750/780 | 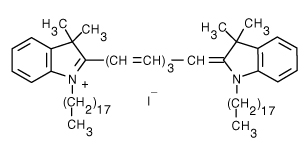 |
| 4-(4-(Dihexadecylamino)styryl)-N-methylpyridinium iodide (DiA) | 456/590 | 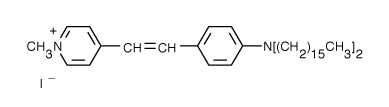 |
| 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2-1,3-benzoxadiazol-4-yl) (ammonium salt) (NBD on head group) | 460/535 | 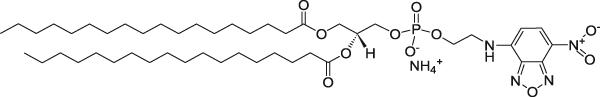 |
| 1-Palmitoyl-2-{12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]dodecanoyl}-sn-glycero-3-phosphocholine (NBD on fatty acid tail) | 460/534 | 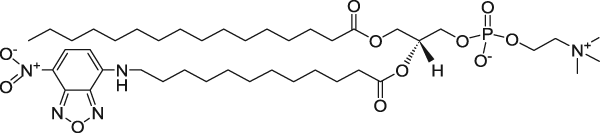 |
| 1,2-Dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (ammonium salt) (Rhodamine lipid) | 560/583 | 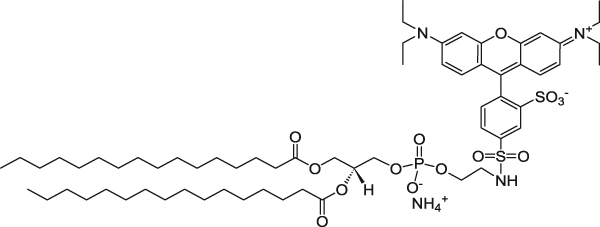 |
| 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine-N-(5-dimethylamino-1-naphthalenesulfonyl) (ammonium salt) (Dansyl lipid) | 336/513 | 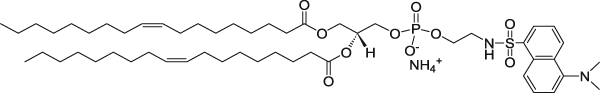 |
| 1,2-Dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(1-pyrenesulfonyl) (ammonium salt) (Pyrene lipid) | 351/379 | 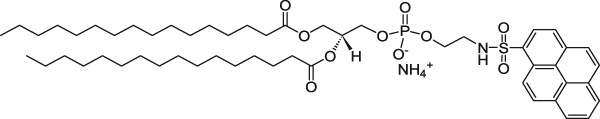 |
| Buffer and Liposome Size | Specification |
|---|---|
| Buffer | Phosphate Buffered Saline |
| pH | 7.4 |
| Liposome Size | 100 nm |
Conjugation Protocol
Materials and Equipment
In order to conjugate your antibody or protein tagged with biotin to ImmunoFluor™-biotin liposomes you will need:
- Laboratory magnetic stirrer is needed for dialysis.
- Vortex laboratory mixer is recommended to have.
- Float-A-Lyzer® with a proper MWCO that easily allows the cleanup of your liposome conjugated ligand from free and non-conjugated protein/peptide/ligand. You need to make sure that the MWCO is below 1,000,000 dalton. At 1,000,000 dalton, the pore size on the dialysis membrane gets close to 100 nm and therefore your liposomes can be dialyzed out. You cannot use dialysis cassettes blindly. Please understand the technique before using either spin column or dialysis cassette. If you do not use the correct MWCO, you can lose your entire prep. For this protocol we recommend MWCO of 300,000 dalton.
Preparation Method
Method A. Two step “Sandwich” protocol- Creating (strept)avidin bridge.
- The total lipid concentration in ImmunoFluor™-Biotin is 22.45 mM. 1% mol of the lipid in liposomes contains biotin group and only half of them are exposed to the outside of the liposomes, which is equal to 0.11 mM of reactive conjugable lipid. For 2 ml volume liposome, this is equal to 2.20×10-7 mol of biotin. Pour ImmunoFluor™-Biotin in a conical tube and vortex it gently with one hand. Use the other hand and slowly add the (strept)avidin solution until the two solutions are mixed. You need to use 10-fold molar excess of (strept)avidin to Biotin lipid. Incubate the solution for 1 h at room temperature.
- Remove the unbound (strept)avidin from the prep by dialysis. We prefer dialysis to size exclusion columns. Dialysis is a much slower process but there will be minimum loss of ImmunoFluor™-Biotin after the prep is cleaned from unbound (strept)avidin. Spin columns are much faster; however, you can easily lose over 50% of the liposomes on the spin column. We recommend using Float-A-Lyzer® dialysis cassette with 300K MWCO from Spectrum Labs. Dialyze the ImmunoFluor™-Biotin/(strept)avidin solution in 1 liter of PBS at pH 7.4 for 8 hours. Change the dialysis buffer with a fresh 1 liter of PBS and let it dialyze for another 8 hours. After this step, ImmunoFluor™-Biotin/(strept)avidin is separated from unbound (strept)avidin.
- Pour ImmunoFluor™-Biotin/(strept)avidin in a conical tube and vortex it gently with one hand. Use the other hand and slowly add the biotinylated antibody or biotinylated ligand solution until the two solutions are mixed. You need to use 2-fold molar excess of biotinylated antibody (ligand) to Biotin lipid. Incubate the solution for 1 h at room temperature.
- Remove the non-conjugated antibody or ligand from the prep by dialysis by using Float-A-Lyzer® dialysis cassette with 300K MWCO from Spectrum Labs. Dialyze the immunoliposome solution in 1 liter of PBS at pH 7.4 for 8 hours. Change the dialysis buffer with a fresh 1 liter of PBS and let it dialyze for another 8 hours. After this step, your cleaned up immunoliposome solution is ready to use.
Method B. Using a antibody/protein/ligand which is already covalently attached to (strept)avidin (less common method)
- The total lipid concentration in ImmunoFluor™-Biotin is 22.45 mM. 1% mol of the lipid in liposomes contains biotin group and only half of them are exposed to the outside of the liposomes, which is equal to 0.11 mM of reactive conjugatable lipid. For 2 ml volume liposome, this is equal to 2.20×10-7 mol of biotin. Pour ImmunoFluor™-Biotin in a conical tube and vortex it gently with one hand. Use the other hand and slowly add the antibody conjugated (strept)avidin until the two solution are mixed. You need to use 2-fold molar excess of antibody conjugated (strept)avidin. Incubate the solution for 1 h at room temperature.
- Remove the non-conjugated antibody or ligand from the prep by dialysis by using Float-A-Lyzer® dialysis cassette with 300K MWCO from Spectrum Labs. Dialyze the immunoliposome solution in 1 liter of PBS at pH 7.4 for 8 hours. Change the dialysis buffer with a fresh 1 liter of PBS and let it dialyze for another 8 hours. After this step, your cleaned up immunoliposome solution is ready to use.
Liposome Particle Calculator
ImmunoFluor™ liposomes are unilamellar and sized to 100 nm. The molar concentration of liposome is 10 mM. By having liposome diameter (nm) and lipid concentration (µM), you can calculate the total number of the lipids in one liposome and the number of the liposomes in one milliliter of the liposome solution. To use the calculator click here.
Technical Notes
- To avoid precipitation of lipid in the non-covalent approach, care needs to be employed in maintaining a high ratio of (strept)avidin to biotin-liposomes. Otherwise, the coupling efficiencies would be relatively low.
- Alternatively, Sepharose® CL-4B size exclusion spin column can be used instead of Float-A-Lyzer®. However keep in mind that a large amount of liposomes will be loss on the column during the process. Dialysis is a much slower process that size exclusion however there will be minimal loss of liposomes.
- If you decide to use a dialysis cassette, you will need to make sure that the MWCO is below 1,000,000 dalton. At 1,000,000 dalton, the pore size on the dialysis membrane gets close to 100 nm and therefore, your liposomes can be dialyzed out. You cannot use dialysis cassettes and spin columns blindly. They come in various sizes and you need to choose the correct size wisely.
- If you are using a ligand or peptide that is hydrophobic then it is recommended to solubilize it in DMSO or DMF and then add the buffer to it. It is recommended not to use more than 5% volume of DMSO or DMF in the solution. DMF and DMSO are both compatible with liposomes and they are also miscible in water. Other organic solvent such as ethanol and chloroform are not compatible with liposomes and will cause the liposomes to lyse. If you end up using DMSO or DMF then after the conjugation reaction is done, you need to remove DMSO and DMF from the liposomes. In order to do that you need to use a dialysis cassette that is made from REGENERATED CELLULOSE MEMBRANE. NOTE: Not all membranes are compatible with DMF and DMSO. We recommend using a Slide-A-Lyzer™ MINI Dialysis Device with MWCO of 2K made from regenerated cellulose membrane manufactured by ThermoFisher. After DMSO or DMF is removed, you can use Float-A-Lyzer® dialysis device for the final step of cleaning up the prep.
- Liposomes should be kept at 4°C and NEVER be frozen.
Database
Direct link to the database page for easy navigation: Immunoliposomes Conjugation Database
Appearance
ImmunoFluor™-Biotin formulation is colored and the color depends on the type of the fluorescent dye that is used (see SDS for appearance). Usually due to the small size of liposomes no settling will occur in the bottom of the vial. The liposomes are packaged in an amber vial.
Ordering/Shipping Information
- All liposome based formulations are shipped on blue ice at 4°C in insulated packages using overnight shipping or international express shipping.
- Liposomes should NEVER be frozen. Ice crystals that form in the lipid membrane can rupture the membrane, change the size of the liposomes and cause the encapsulated drug to leak out. Liposomes in liquid form should always be kept in the refrigerator.
- Clients who order from outside of the United States of America are responsible for their government import taxes and customs paperwork. Encapsula NanoSciences is NOT responsible for importation fees to countries outside of the United States of America.
- We strongly encourage the clients in Japan, Korea, Taiwan and China to order via a distributor. Tough customs clearance regulations in these countries will cause delay in custom clearance of these perishable formulations if ordered directly through us. Distributors can easily clear the packages from customs. To see the list of the distributors click here.
- Clients ordering from universities and research institutes in Australia should keep in mind that the liposome formulations are made from synthetic material and the formulations do not require a “permit to import quarantine material”. Liposomes are NOT biological products.
- If you would like your institute’s FedEx or DHL account to be charged for shipping, then please provide the account number at the time of ordering.
- Encapsula NanoSciences has no control over delays due to inclement weather or customs clearance delays. You will receive a FedEx or DHL tracking number once your order is confirmed. Contact FedEx or DHL in advance and make sure that the paperwork for customs is done on time. All subsequent shipping inquiries should be directed to Federal Express or DHL.
Storage and Shelf Life
Storage
ImmunoFluor™ products should always be stored at in the dark at 4°C, except when brought to room temperature for brief periods prior to animal dosing. DO NOT FREEZE. If the suspension is frozen, the encapsulated drug can be released from the liposomes thus limiting its effectiveness. In addition, the size of the liposomes will also change upon freezing and thawing.
Shelf Life
ImmunoFluor™-Biotin is made on daily basis. The batch that is shipped is manufactured on the same day. It is advised to use the products within 2 months of the manufacturing date.
References and background reading
1. Hermanson GT. Bioconjugate techniques. Academic press; 2013 Jul 25.
3. Haugland RP, Bhalgat MK. Preparation of avidin conjugates. Immunochemical Protocols. 1998:185-96.











