DOPC:DOPS Liposomes Containing Calcein or Carboxyfluorescein
Description
Fluorescent leakage assay is a method that is extensively used for measuring the disruption of the liposomal membrane. The disruption can be due to various external triggers such as the interaction of molecules (e.g. peptides, proteins, detergents, etc.) with the surface of the liposomes. The liposomal membrane can also be disrupted when external energy is absorbed by the system, such as exposing the liposomes to sonic sound, mechanical pressure, or heat.
When liposomes are disrupted, their contents will leak out. If you investigate old literature, you will see that in a large percentage of fluorescent leakage assays, the experiments involve encapsulating a self-quenching concentration of fluorescent dyes, such as calcein and carboxyfluorescein. The problem with calcein and carboxyfluorescein is that the liposomes have to be made very fresh and be used right away.
These liposomes are formulated upon ordering. Before placing the order, make sure that your experimental setup is ready and the formulation can be used immediately.
The dye encapsulated liposomes are not separate from the free fluorescent dye. Be sure to have the proper spin column for cleaning up the prep.
We will send you the buffer with adjusted osmolarity for washing the column and eluting the liposomes. You will also get a 0.2 % Triton solution for total lysis of the liposomes.
Technical Information
DOPC:DOPS Liposomes Containing Calcein or Carboxyfluorescein
For more information on the lipid composition of the liposomes mentioned above click here.
| Fluorescent Dye | Excitation/Emission (nm) |
|---|---|
| 5(6)-Carboxyfluorescein | 492/517 nm |
| Calcein | 470/509 nm |
| *You can choose one of these two dyes to be encapsulated inside the liposomes. | |
| Buffer and Liposome Size | Specification |
|---|---|
| Buffer | 20 mM HEPES |
| pH | 7.4 |
| Liposome Size | 100 nm |
| * If you prefer different buffers, please specify in your order. | |
Cleaning Protocol- Separation of the dye encapsulated liposomes from the free dye
Materials and Equipment
In order to clean up the liposomes and separate the dye encapsulated liposomes from the free dye you will need the following:
- Laboratory mini/micro centrifuge that can be used with Eppendorf tubes.
- Size exclusive spin column. The success of your experiment depends on correctly removing the non-encapsulated dye from the dye encapsulated liposomes using the spin column. We strongly recommend using Amershan Microspin G-50 columns by Cytiva. Not every commercial G-50 column is the same. We have noticed that with some brands we can not achieve the level of cleanness that is desired.
In order to use the spin column, please do the following:
- Cut the bottom of the spin column.
- Drain the buffer in the tube by microfugation.
- Add 0.5 mL of HEPES buffer to the resin, and wash and drain the buffer by microfugation. We will send HEPES buffer to you in a separate tube. This buffer has the exact same osmolarity as the 200 mM 5,6-Carboxyfluorescein or calcein. There is no need to super dry the column or run the microcentrifuge for a very long time. A total of 30-45 seconds will be sufficient.
- Add 10 uL of liposomes to the middle of the resin. The pipette tip should touch the center of the resin surface.
- Make sure NOT to touch the wall of the tube with the pipette tip. Doing this will cause a large percentage of the liposomes to fall in the space between the resin and the wall. As a result, those liposomes will pass to the bottom disk of the column without being separated from the un-encapsulated dye. This will significantly decrease the sensitivity of your assay.
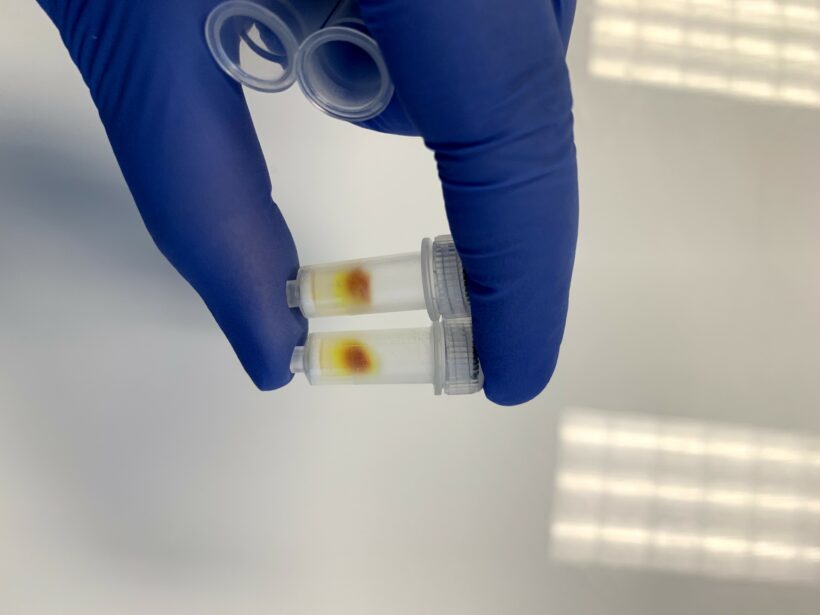
Figure 1 shows an example of the column being used correctly. You can see a large dark spot on top of the column and traces of light yellow going down the column. The solution that comes out to the collection tube should be slightly yellow.
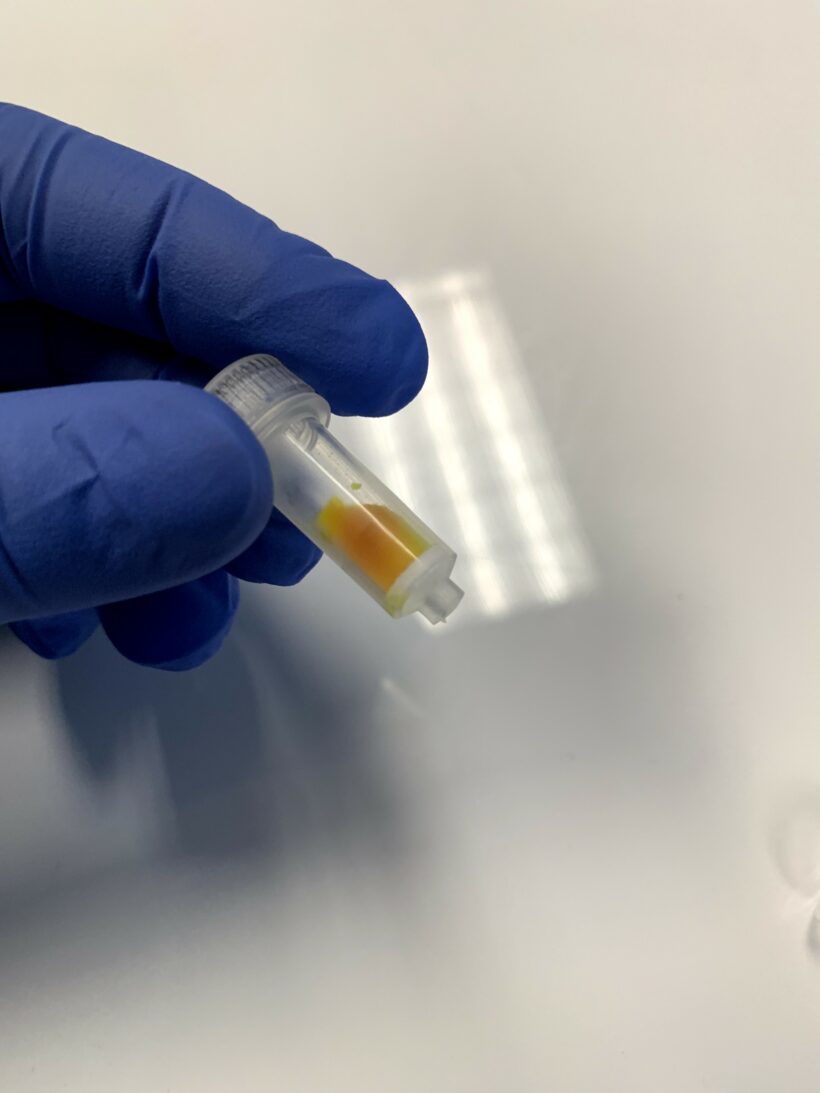
The clean liposomes are light yellow in the collection tube as shown in Figure 2.

Figure 3 shows an example of when the column is used inaccurately. The pipette tip touched the wall when pipetting the liposomes. Liposomes got into the empty space between the resin and the wall, and the entire column was then colored. When the column is used correctly, only one spot on top is dark, not the whole column.
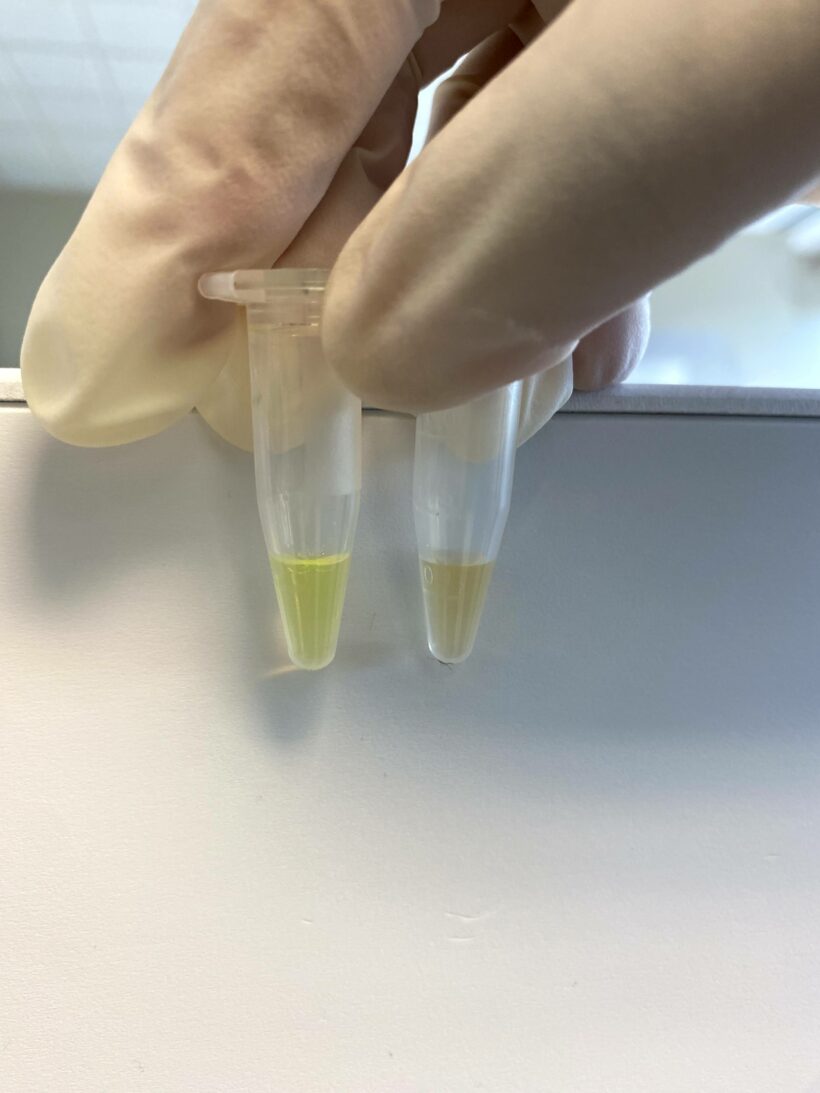
10 uL of liposome solution was added to the column, and 10 uL of cleaned liposomes was collected from the tube.
10 uL of cleaned liposomes is diluted by 40 fold by adding 390 uL of HEPES buffer. 400 uL of cleaned liposomes is divided into two 200 uL parts.
*Please keep in mind that the dilution factor depends on many factors such as your lipid composition and your fluorometer. If your fluorometer shows overload/above range error then you need to dilute your solution even more. We will do the assay on our side with your liposomes and will inform you about the proper dilution factor at the time of shipping your liposomes.
Part 1 vial: Measure the fluorescence on your fluorometer. The fluorescence is low.
Part 2 vial: Add 5 uL of 0.2% Triton solution (we will send you this solution) and vortex for 1 minute. Measure the fluorescence. Fluorescent dye is leaked out, and the dye is de-quenched. Your solution color should change very slightly to a greenish color. After measuring the fluorescence using the fluorometer the fluorescence should jump higher.
Depending on the lipid composition and the degree of cleanness of the prep the fluorescent should jump between 5-15 folds.
Any type of disruption to the membrane will cause leakage, and the number on the fluorometer will read much higher than the intact liposomes. If your molecules disrupt the membrane, you should easily observe an increase in fluorescence.
Technical Notes
- Liposomes are unilamellar and sized to 100 nm. If you need them to be made in another size, then you should mention that at the time of ordering. Unilamellar liposomes can be made in 50 nm and 200 nm sizes. Liposomes that are larger than 200 nm are multilamellar structure.
- Liposomes are made in HEPES buffer at pH 7.4 but it can be made in any other buffer of your choice. You can specify your buffer at the time of ordering.
- Liposomes are made in degassed buffer that is purged with argon to avoid oxidation of the unsaturated phospholipids.
- Liposomes are made under sterile condition.
- Liposomes are unilamellar and therefore due to their small size they will not settle in the bottom of the vial.
- If you need to take multiple aliquots out of the vial, for use over a period of time, then it is advised to take extreme care in not contaminating the vial. It is recommended to handle the vial under a sterile hood to maintain the sterility of the product.
- Liposomes should never be frozen. Ice crystals that form during freezing will rupture the lipid membrane of the liposomes and change the size the liposomes particles.
- Liposomes should be kept in refrigerator at 4°C in order to avoid the hydrolysis of the liposomes.
- Liposomes should be kept at 4°C and NEVER be frozen.
Appearance
Liposomes containing calcein or carboxyfluorescein formulation is a yellow translucent liquid. Usually due to the small size of liposomes no settling will occur in the bottom of the vial. The liposomes are packaged in an amber vial.
Ordering/Shipping Information
- All liposome based formulations are shipped on blue ice at 4°C in insulated packages using overnight shipping or international express shipping.
- Liposomes should NEVER be frozen. Ice crystals that form in the lipid membrane can rupture the membrane, change the size of the liposomes and cause the encapsulated drug to leak out. Liposomes in liquid form should always be kept in the refrigerator.
- Clients who order from outside of the United States of America are responsible for their government import taxes and customs paperwork. Encapsula NanoSciences is NOT responsible for importation fees to countries outside of the United States of America.
- We strongly encourage the clients in Japan, Korea, Taiwan and China to order via a distributor. Tough customs clearance regulations in these countries will cause delay in custom clearance of these perishable formulations if ordered directly through us. Distributors can easily clear the packages from customs. To see the list of the distributors click here.
- Clients ordering from universities and research institutes in Australia should keep in mind that the liposome formulations are made from synthetic material and the formulations do not require a “permit to import quarantine material”. Liposomes are NOT biological products.
- If you would like your institute’s FedEx or DHL account to be charged for shipping, then please provide the account number at the time of ordering.
- Encapsula NanoSciences has no control over delays due to inclement weather or customs clearance delays. You will receive a FedEx or DHL tracking number once your order is confirmed. Contact FedEx or DHL in advance and make sure that the paperwork for customs is done on time. All subsequent shipping inquiries should be directed to Federal Express or DHL.
Storage and Shelf Life
Storage
Liposomes containing calcein or carboxyfluorescein should always be stored at in the dark at 4°C, except when brought to room temperature for brief periods prior to animal dosing. DO NOT FREEZE. ENS is not responsible for results generated by frozen product.
Shelf Life
Liposomes containing calcein or carboxyfluorescein are made on daily basis. The batch that is shipped is manufactured on the same day. It is advised to use the products within 30 days of the manufacturing date.











